Getty Images
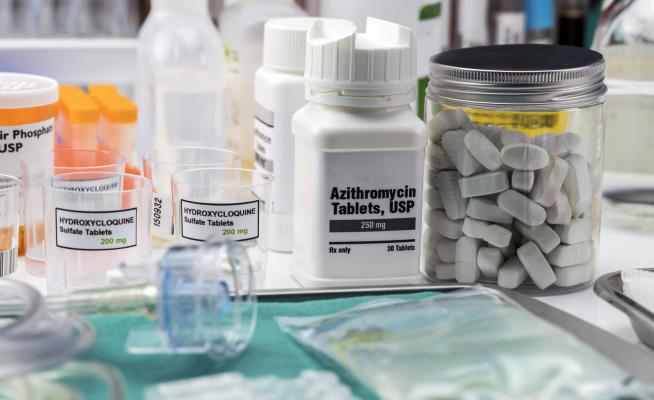
September 17, 2020 — Debates over whether hydroxychloroquine should be taken to help lessen the duration and impact of COVID-19 (SARS-CoV-2) have revolved around the drug’s reputation for causing abnormal heart rhythms and cardiac arrest. Because of this, the U.S. Food and Drug Administration (FDA) revoked its emergency use authorization for the drug in treating COVID-19.
Another drug, azithromycin, a commonly-prescribed antibiotic, also is being investigated as a potential treatment for COVID-19. Azithromycin’s association with cardiac events also has been debated. In 2013, the FDA issued a warning for azithromycin stating that it had been linked to cardiac events, but subsequent studies have yielded mixed results.
Now, researchers from the University of Illinois Chicago have found that azithromycin by itself is not associated with an increase in cardiac events. However, if the drug is taken with certain other drugs that affect the electrical functioning of the heart, then cardiac events increased.
“Our findings should give researchers and clinicians looking at azithromycin as a potential treatment for COVID-19 pause,” said Haridarshan Patel, a researcher in the department of pharmacy systems, outcomes and policy at the UIC College of Pharmacy and corresponding author on the paper. “We found that if taken together with drugs that affect the electrical impulses of the heart, the combination is linked with a 40% increase in cardiac events, including fainting, heart palpitations and even cardiac arrest.”
Their findings are published JAMA Network Open in the article "Comparison of Cardiac Events Associated With Azithromycin vs Amoxicillin."[1]
Drugs that affect the electrical impulses of the heart, specifically the interval in the electrocardiogram (ECG) called the QT interval, are called QT-prolonging drugs. These drugs include blood pressure medications such as ACE inhibitors and beta-blockers, some antidepressants, anti-malaria drugs such as hydroxychloroquine and chloroquine, opioid medications and even muscle relaxers.
“Because QT-prolonging drugs are used so commonly, our findings suggest that doctors prescribing azithromycin should be sure that patients are not also taking a QT-prolonging drug,” Patel said.
In a previous study, Patel and colleagues found that one in five people prescribed azithromycin also were taking a QT-prolonging drug.
Previous studies looking at azithromycin and cardiac events examined specific populations that tend to be older and have more health issues, including Medicaid patients and veterans. But in this study, Patel and colleagues used a large database containing medical data on millions of patients in the United States with a mean age of 36 years old.
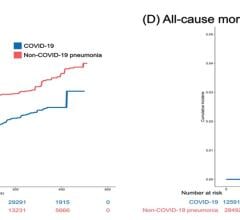
The risk of cardiac events with azithromycin was evaluated against amoxicillin, another antibiotic that has never been linked to cardiac events and which has no impact on the QT-interval. The researchers looked at data from more than 4 million patients enrolled in private health insurance plans who were hospitalized or visited an emergency department for a cardiac event between 2009 and 2015 who started taking either amoxicillin or azithromycin within five days of their hospital visit. There were approximately 2 million episodes in each group. Cardiac events included ventricular arrhythmias, fainting, palpitations and cardiac arrest, and death.
“Drugs often prolong QT-interval but may not necessarily result in cardiac events that self-resolve over time,” Patel said. “We looked at events that led to emergency department visits or hospitalizations in this study.”
The researchers found that the likelihood of cardiac events with azithromycin compared with amoxicillin were not significantly higher, and these events actually were quite low or rare in both groups, with the most common cardiac events being fainting and palpitations. However, among patients taking both a QT-prolonging medication and azithromycin together, the risk of cardiac events was 40% higher compared with the amoxicillin group.
“Because both QT-prolonging drugs and azithromycin are so commonly prescribed, the risk for cardiac events due to the combination, while still rare, is serious,” Patel said. “Studies looking at using azithromycin to treat COVID-19 or other diseases should very carefully consider its use among patients who are also taking QT-prolonging medications.”
Gregory Calip, Robert DiDomenico, Glen Schumock and Todd Lee of the UIC College of Pharmacy and Katie Suda of the University of Pittsburgh are co-authors on the study.
Related Content on Cardiac Issues Related to COVID-19 Treatments:
VIDEO: Why QT-prolongation Occurs in COVID-19 Patients on Hydroxychloroquine and Azithromycin — Interview with Andrew D. Krahn, M.D.,
VIDEO: Overview of Hydroxychloroquine and FDA Warning in its use to Treat COVID-19 — Interview with Marianne Pop, Pharm.D.
FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine for COVID-19
COVID-19 Hydroxychloroquine Treatment Brings Prolonged QT Arrhythmia Issues
FDA Reports of Deaths and Injuries From Use of Antimalarial hydroxychloroquine in COVID-19 Patients
VIDEO: Cardiologists Manage Trial Testing if Hydroxychloroquine Protects Clinicians From COVID-19 — Interview with William O'Neill, M.D.
First Large-scale U.S. Study on Hydroxychloroquine COVID-19 Prophylaxis Begins in Detroit
Reference:

 March 20, 2024
March 20, 2024