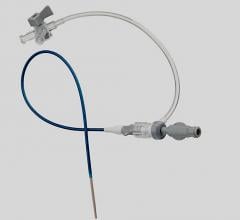
November 4, 2009 – Cordis and FDA notified healthcare professionals this week of a nationwide recall of all lots of the CROSSOVER Sheath Introducer.
The long-coil reinforced, kink-resistant catheter sheath is intended for use in arterial and venous procedures requiring the percutaneous introduction of therapeutic or diagnostic intravascular devices or fluids. The recall was due to stretching or fracture of the sheath during use.
The FDA said in the event of a device fracture, separated segments of the device can embolize downstream in the bloodstream and impede blood flow distal to the point where it lodges, resulting in ischemia or infarct to the distal extremity. Since this device is coil reinforced, any separation of the cannula has the potential to expose portions of the coil creating the potential for vessel dissection or perforation. Unplanned open surgery may be required to remove the retained segments or control bleeding.
For more information: www.fda.gov/Safety/MedWatch


 June 10, 2020
June 10, 2020