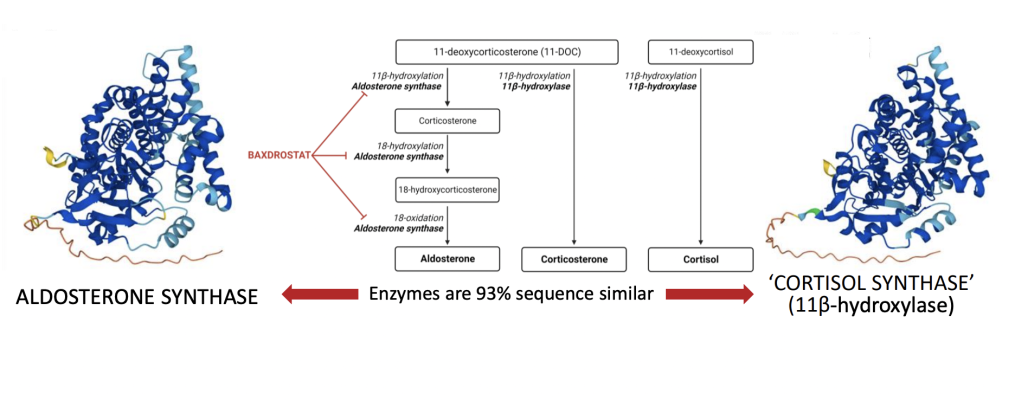
November 9, 2022 — CinCor Pharma, Inc. announced the presentation today of Phase 2 data from its BrigHtn trial as part of the late-breaking science session at the 2022 American Heart Association (AHA) Scientific Sessions. Baxdrostat is a highly selective, once daily oral small molecule inhibitor of aldosterone synthase.
“We were honored to present our Phase 2 BrigHtn data as part of a late-breaking session focused on new treatments for the increasingly recognized unmet needs in resistant hypertension at this year’s AHA conference,” said Mason Freeman, M.D, Chief Medical Officer at CinCor. “Baxdrostat significantly decreases aldosterone in plasma and urine, while increasing plasma renin as a physiological compensatory change. These changes in aldosterone and renin following treatment with baxdrostat reflect less salt exposure to the kidney and reduced blood pressure over time. And, unlike earlier generations of aldosterone synthase inhibitors, serum cortisol levels were not reduced, once again confirming the highly selective mechanism of baxdrostat. Collectively these findings demonstrate how baxdrostat uniquely helps address a known cause of elevated blood pressure, potentially offering a much needed treatment option for patients with tough to control hypertension.”
BrigHtn Trial Highlights
Aldosterone and Renin Activity Reinforce the Biological Mechanism of Baxdrostat
- Dose-dependent reduction in both plasma and urine aldosterone support inhibition of aldosterone synthase and the mechanism of action of baxdrostat
- Urine aldosterone levels 24-hours post-treatment quantitatively measure total body aldosterone production with less variation than plasma to provide direct evidence of baxdrostat’s aldosterone lowering capabilities
- Dose-dependent changes in renin activity support physiological salt response
- No meaningful impact on cortisol confirms baxdrostat is highly selective for aldosterone synthase
Clinically Meaningful and Dose-Dependent Reduction in Blood Pressure With Baxdrostat
- BrigHtn successfully met its primary endpoint, demonstrating a statistically significant change from baseline in mean seated systolic blood pressure (SBP) versus placebo:
- Dose-dependent reductions in SBP of −20.3 mmHg (2-mg), −17.5 mmHg (1-mg), and −12.1 mmHg (0.5-mg)
- Statistically significant placebo-adjusted decreases of −11.0 (2-mg, P = 0.0001) and −8.1 (1-mg, P = 0.003)
- Secondary endpoint results included baxdrostat significantly lowering diastolic blood pressure (DBP) by 5.2 mmHg in the 2mg dose arm, and approximately 46% of patients in the 2 mg dose arm achieving blood pressure control (SBP less than 130mmHg)
- Patient demographics and baseline characteristics were diverse and well-balanced across treatment arms
Clinical Safety and Tolerability of Baxdrostat Support a Well-Tolerated Profile
- No drug related serious adverse events (SAEs) observed or major safety concerns were reported across all three dose cohorts tested after 12 weeks of treatment
- Transient and manageable adverse events of special interest included hypotension, hyponatremia, or elevated potassium levels
- Low discontinuation rate of less than 1% (2 patients) due to transient treatment-related adverse events; all patients completed the study on drug
- Well-tolerated profile and differentiated mechanism of action supports combination approaches
The BrigHtn trial was a Phase 2 randomized, double-blind, placebo-controlled dose-ranging clinical trial designed to assess the safety and efficacy of baxdrostat in subjects who have not achieved their target blood pressure despite receiving three or more antihypertensive agents at their maximally tolerated doses, one of which must be a diuretic. The trial evaluated three active doses of baxdrostat (0.5 mg, 1.0 mg, and 2.0 mg) compared to placebo control in 275 patients randomized across all four dosing cohorts, with 248 patients completing. The primary endpoint of BrigHtn was the change in mean seated SBP from randomization to trial end after 12 weeks of treatment.
For more information: www.cincor.com


 November 14, 2025
November 14, 2025