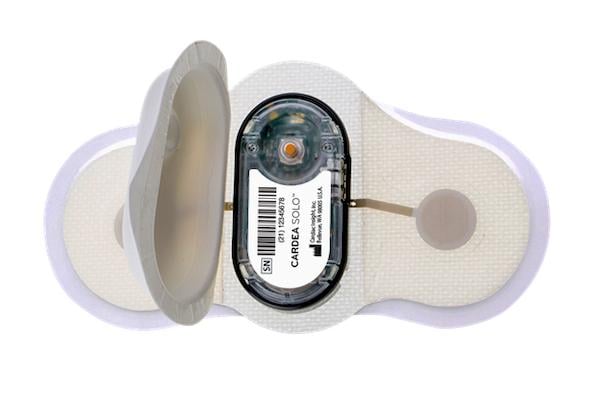
Cardiac Insight's Heart@Home ECG Test Kit can help address new telehealth demands placed on cardiology practices by the COVID-19 response using arrhythmia diagnosis technology of the wearable Cardea Solo Sensor.
May 4, 2020 — Cardiac Insight Inc., a U.S.-based digital healthcare innovator specializing in body-worn sensor technology and automated electrocardiogram (ECG)-analysis software, announced the Heart@Home ECG Test Kit can help address new telehealth demands placed on cardiology practices by the COVID-19 response.
The kit enables physicians to prescribe the proven arrhythmia diagnosis technology of the Cardea Solo Sensor which is express-shipped directly from Cardiac Insight to the patient’s home, along with easy-to-follow instructions. The patient simply mails the single-use sensor to the cardiologist’s office in the included shipping envelope after wearing the sensor for the prescribed time period.
Clinicians can immediately retrieve data from the device to inform diagnoses using Cardea Solo’s smart cable and robust, proprietary algorithm-based ECG software, provided free of charge with the purchase of the Heart@Home ECG Test Kit. The entire process in the office takes less than five minutes to create a complete ECG report.
“In these unprecedented times, while legacy ECG service companies employing decades-old technology are scaling back operations and decreasing services, Cardiac Insight is innovating and acting quickly to provide cardiologists and their patients with essential and easy-to-use telehealth options. As a single-use sensor, Cardea Solo affords patients, doctors and clinical staff more infection control protections with no need for cleaning or transferring devices between patients,”said Robert Odell, president and chief operating officer of Cardiac Insight. “We believe the new Heart@Home program further strengthens the physician and patient relationship in a time when trust and privacy is more critical than ever, by fostering direct interactions and removing third-party data analysis vendors from the equation.”
The Cardea Solo ECG System allows physicians to reduce the diagnostic timeline of patients potentially at risk of cardiac arrhythmias from weeks to just days. These arrhythmias include atrial fibrillation (AFib). If left undiagnosed, AFib can lead to debilitating stroke, heart failure and sudden cardiac death.
The Cardea Solo Heart@Home ECG Test Kit is currently available for office-based practices and hospital clinics in the U.S. and is designed to address the needs of Electrophysiologists, Cardiologists, Internal Medicine and Family Medicine physicians. Comprehensive program information and telehealth resources are available on request here.
For more information: www.cardiacinsightinc.com


 December 19, 2025
December 19, 2025 









