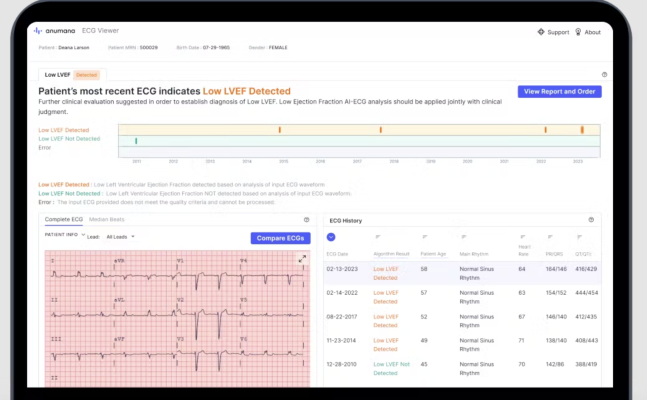
Cambridge, MA-based artificial intelligence (AI)-driven health technology company, Anumana, has announced its receipt of the International Organization for Standardization (ISO) 13485 certification for its Quality Management System. ECG-AI LEF, the company’s breakthrough AI algorithm, received U.S. Food and Drug Administration (FDA) clearance in September 2023. Image courtesy: Anumana
May 21, 2024 — Anumana, a Cambridge, MA-based artificial intelligence (AI)-driven health technology company and portfolio company of nference, has announced it has received the International Organization for Standardization (ISO) 13485 certification for its Quality Management System.
Anumana’s software-as-a-medical device (SaMD) ECG-AITM solutions aim to detect hidden diseases using standard-of-care ECG readings, enabling clinicians to enhance and improve care with real-time AI insights. ECG-AI LEF, the company’s breakthrough AI algorithm using routine 12-lead ECG data to detect Low Ejection Fraction (LEF), a commonly undiagnosed indicator of heart failure,1 received U.S. Food and Drug Administration (FDA) clearance in September 2023, and is currently under review in Europe.
This certification, the quality management standard for device manufacturers, indicates that a company has developed robust policies and procedures for the development and manufacture of regulated medical devices, according to a written statement released by the Cambridge, MA-based company.
Anumana received ISO 13485 certification following an independent third-party certification firm’s rigorous assessment of its Quality Management System. With this certification, Anumana strengthens its ability to provide software-as-a-medical device (SaMD) ECG-AI algorithms that consistently meet customer and regulatory requirements. The company reports that its cutting edge AI platform solutions “unlock the language of the heart by harnessing the power of its electrical signal to transform cardiac care.”
“Anumana’s ISO13485 certification is a testament to our commitment to excellence in the development and manufacturing of regulated medical devices that adhere to international standards and requirements,” said David McMullin, Anumana’s Chief Business Officer. “As a rapidly growing international medical device manufacturer, this milestone underscores our leadership in pioneering and bringing to market clinically validated and regulated ECG-AI algorithms as SaMDs, validates our capabilities, and enables Anumana to extend the reach of our technology to clinicians and patients worldwide,” McMullin added.
The company was founded by nference in collaboration with Mayo Clinic to leverage the clinical and technical expertise of both organizations to develop innovative ECG-AI technology into a clinically meaningful, medical-grade, and easy to use tool for clinicians to advance patient care. Anumana’s software-as-a-medical device (SaMD) ECG-AITM solutions aim to detect hidden diseases using standard-of-care ECG readings, enabling clinicians to enhance and improve care with real-time AI insights.
More information: www.anumana.ai
Reference:
1 Jaskanwal D Sara, Takumi Toya, Riad Taher, Amir Lerman, Bernard J Gersh, Nandan S Anavekar. Asymptomatic Left Ventricle Systolic Dysfunction. European Cardiology Review 2020, 15:e13; https://doi.org/10.15420/ecr.2019.14.


 January 15, 2026
January 15, 2026 









