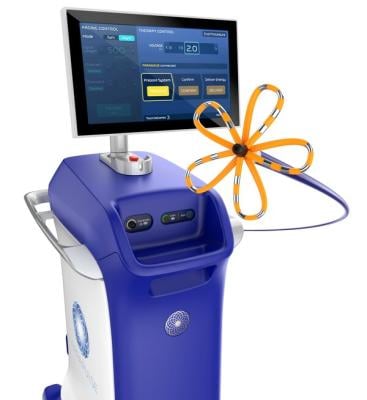
The U.S. Food and Drug Administration (FDA), has granted approval to Boston Scientific for its FARAPULSE Pulsed Field Ablation System. A company statement reported that its PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., intermittent) atrial fibrillation (AF) and is a unique new alternative to standard-of-care thermal ablation treatment. Image courtesy: Boston Scientific
January 31, 2024 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE Pulsed Field Ablation (PFA) System. The FARAPULSE PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., intermittent) atrial fibrillation (AF) and is a unique new alternative to standard-of-care thermal ablation treatment.
"The approval of the FARAPULSE PFA System marks an important milestone for the millions of people living with paroxysmal AF and is an incredible opportunity to bring the first PFA system designed and built solely for this type of ablation therapy to physicians in the U.S.," said Nick Spadea-Anello, president, Electrophysiology, Boston Scientific. In the written statement, he added, "A high bar has been set by performance of the system in clinical and commercial settings – where more than 40,000 patients have been treated to date – and we look forward to continuing to lead the way with this differentiated technology in the growing PFA space."
The Boston Scientific announcement also reported the following: During a traditional ablation procedure, a catheter is guided to the interior of the heart and generates extreme temperatures – hot or cold – to destroy targeted areas associated with abnormal heart rhythms. The FARAPULSE PFA System, however, relies on tissue-selective, non-thermal electric fields to ablate heart tissue and avoid damage to surrounding structures. Positive 12-month data from the pivotal ADVENT clinical trial – the first randomized clinical trial to directly compare the efficacy and safety of the system against standard-of-care ablation, published in the New England Journal of Medicine Nov. 2, 2023 – found that therapy with the device was as safe and effective as conventional thermal ablation, with statistically shorter ablation times and a quick learning curve for physicians. Additional real-world data from more than 17,000 patients in the MANIFEST-17K registry demonstrated continued real-world safety of the system, with no reports of permanent phrenic nerve palsy, pulmonary vein stenosis or esophageal injury.
"Within the ADVENT clinical trial, the FARAPULSE PFA System was shown to be a safe, effective and efficient option for treating paroxysmal AF, and extensive global real-world use has mirrored that profile," said Vivek Reddy, M.D., director of electrophysiology, Mount Sinai Fuster Heart Hospital, New York. "Tissue preferentiality and long-term efficacy, combined with markedly shorter procedure times and learning curves, position the FARAPULSE PFA System with strong potential to become a practice-changing technology for both U.S. physicians and patients alike," Reddy added.
According to the company, the FARAPULSE PFA System delivers pulsed field energy and consists of the FARAWAVE Ablation Catheter, the FARASTAR Ablation Generator, and the FARADRIVE Steerable Sheath, which is complemented by the VersaCross Connect Access Solution for the FARADRIVE Steerable Sheath to provide safe and efficient access to the left side of the heart during procedures with the system. The FARAWAVE catheter is used to treat a range of pulmonary vein anatomies using an over-the-wire catheter with variable basket and flower shapes, allowing the device to adapt to individual patient anatomies. These configurations reinforce ease-of-use for physicians and promote reproducible procedures between operators.
Boston Scientific completed enrollment in the first phase of the ADVANTAGE AF clinical trial in the third quarter of 2023, which is studying the system for the treatment of patients with drug-refractory, symptomatic, persistent AF, and commenced enrollment in a second phase of the study to evaluate the safety and effectiveness of adjunctive use of the FARAPOINT PFA Catheter for cavotricuspid isthmus (CTI) ablations, a procedure used to treat atrial flutter. The company also recently commenced the AVANT GUARD clinical trial to evaluate the safety and efficacy of the system as a first-line treatment for persistent AF compared to anti-arrhythmic drug therapy.
The FARAPULSE PFA System was granted Breakthrough Device Designation from the Center for Devices and Radiological Health (CDRH) of the U.S. FDA in 2019 and received CE Mark in 2021. Boston Scientific plans to immediately launch the system in the U.S. The company is developing a navigation-enabled version of the FARAWAVE catheter alongside the FARAVIEW Software Module and anticipates regulatory approval in 2024.
More information: www.bostonscientific.com
Related content:


 January 15, 2026
January 15, 2026 









