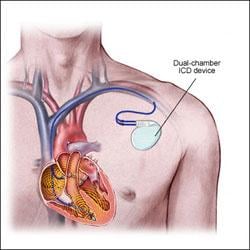
April 28, 2010 — The U.S. District Court in Minnesota this week declined to accept the plea agreement between Guidant and the U.S. Justice Department related to its failure to report adverse events from its implantable cardioverter defibrillators (ICDs) in 2005. Boston Scientific acquired Guidant after the incident.
The court invited the parties to consider a modified agreement fashioned to further serve the public interest, including community service, public education and charitable activities, and suggested the Justice Department allocate a portion of the settlement funds to Medicare. The court did not suggest revisions to the remaining terms of the plea agreement.
Boston Scientific said it plans to work with the DOJ in an effort to develop a modified plea agreement that is acceptable to the Court, the Justice Department and the company.
In November, Guidant agreed to plead to two misdemeanor charges related to the failure to include information in reports to the U.S. Food and Drug Administration, and Boston Scientific agreed to pay $296 million on behalf of Guidant.
For more information: www.bostonscientific.com


 January 13, 2026
January 13, 2026 









