March 8, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech announced today the first cases with its investigational THERMOCOOL SMARTTOUCH SF Dual Energy Catheter took place as part of the SmartfIRE Study. The first procedure was performed by Dr. Tom De Potter at OLV Hospital in Aalst, Belgium. Several additional cases were performed by Dr. Mattias Duytschaever at AZ Sint-Jan Brugge Oostende AV in Brugge, Belgium, which is also taking part in the clinical trial.
SmartfIRE is a pivotal, prospective, multi-center, single-arm study that will enroll approximately 135 patients in Europe to evaluate the safety and effectiveness of Biosense Webster’s dual energy pulsed field (PF) / radiofrequency (RF) ablation system. The study will evaluate the system for the treatment of drug refractory symptomatic paroxysmal atrial fibrillation (AFib) during standard electrophysiology mapping and ablation procedures.
“While radiofrequency ablation has decades of safety and efficacy data to support it, pulse field ablation is a novel technology that shows promise in advancing the safety and ease of catheter ablation procedures; the versatility of a dual-energy catheter would give electrophysiologists the choice of using either one or both modalities at their fingertips,” said Tom De Potter, M.D., FEHRA, Associate Director, Cardiovascular Center, Department of Cardiology, Electrophysiology Section, OLV Hospital, Aalst, Belgium, who conducted the first case. “In caring for patients with AFib, my goal is to deliver a treatment that is maximally safe and efficient at the same time, and this dual-energy system may give me the ability to switch between radiofrequency and pulsed field and customize treatment during a procedure to deliver the most appropriate energy.’’
Catheter ablation is a minimally invasive procedure performed by an electrophysiologist to treat heart rhythm disorders, including AFib, by interrupting irregular electrical pathways in the heart by delivering either heat (RF ablation) or cold (Cryoablation).2 PF ablation represents a new approach to treating AFib, utilizing a controlled electric field to selectively ablate cardiac tissue that causes the irregular heartbeat through a process called irreversible electroporation (IRE). Because the energy does not rely on thermal effects to ablate target cardiac tissue, IRE offers the potential to reduce the risk of damage to surrounding tissues including esophageal, pulmonary vein, and phrenic nerve injury.3
AFib is the most common type of cardiac arrhythmia and impacts nearly 40 million people worldwide,4 and 11 million people in Europe alone.5 Today, about 1 in 4 adults over the age of 40 are at risk for developing AFib.6 Despite these projections, many people are unfamiliar with AFib symptoms, available treatment options and the importance of early treatment to avoid disease progression.7
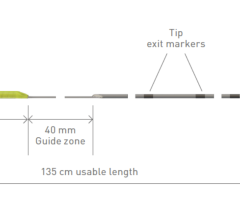
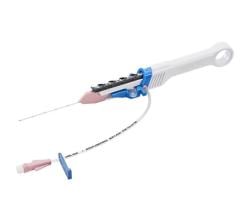
The principal components of the ablation system to be evaluated in this investigation are the THERMOCOOL SMARTTOUCH SF Dual Energy Catheter and the TRUPULSE Generator. The THERMOCOOL SMARTTOUCH SF Bi-Directional Navigation Catheter with Contact Force Sensing Capability, is a steerable, multi-electrode luminal, irrigated catheter. It is CE marked for use in catheter-based cardiac electrophysiological mapping (stimulating and recording) and, when used with a RF generator, for cardiac ablation. The investigational TRUPULSE Generator provides both the RF energy and the novel uni-polar, bi-phasic pulse sequence to the catheter through the toggling of the two energy sources on the generator monitor.8 The THERMOCOOL SMARTTOUCH SF Catheter is to be used in this investigational study with the TRUPULSE Generator, to transmit the PF or RF energy to the catheter tip electrode for cardiac ablation. The catheter and the generator are integrated with the CARTO 3 System. The THERMOCOOL SMARTTOUCH SF Dual Energy Catheter and the TRUPULSE Generator are not available for sale in any region.
“Enrollment of the first patient in the SmartfIRE Study is an important milestone in Biosense Webster’s journey to harness the latest science and technology to improve the treatment of AFib around the world,’’ said Celine Martin, Company Group Chairman, Cardiovascular & Specialty Solutions Group, Johnson & Johnson. “As a long-standing leader in the field of cardiac ablation, we are working to bring forward a versatile, best-in-class portfolio of pulsed field ablation solutions – complementary to our radiofrequency ablation catheter portfolio – to address various ablation strategies in the treatment of atrial arrhythmias.”
The SmartfIRE Study will contribute to a growing body of evidence being developed by Biosense Webster in support of PF ablation as a safe and effective option for AFib treatment. In February 2023, the company announced early success in the inspIRE clinical trial – a prospective, multi-center, single-arm study conducted in Europe to evaluate the safety and efficacy of the VARIPULSE Catheter and TRUPULSE™ Generator in treating drug refractory recurrent symptomatic paroxysmal AFib.8 In April 2022, the company announced that the first patients were enrolled in admIRE – a prospective, multi-center, single-arm study of approximately 400 patients in the U.S. to evaluate the safety and effectiveness of the VARIPULSE™ Catheter and the TRUPULSE Generator for the treatment of refractory symptomatic paroxysmal AFib during standard electrophysiology mapping and ablation procedures.
For more information: www.biosensewebster.com
References:
- Johnson & Johnson MedTech comprises the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson’s MedTech segment.
- OLV Hospital entered into a clinical trial agreement with Johnson & Johnson Medical NV/SA for its participation in the SmartfIRE Study. Dr. Tom De Potter serves as a study investigator and was not compensated for his contributions to this announcement.
- AZ Sint-Jan Brugge Oostende AV entered into a clinical trial agreement with Johnson & Johnson Medical NV/SA for its participation in the SmartfIRE Study. Dr. Mattias Duytschaever serves as a study investigator and was not compensated for his contributions to this announcement.
- ClinicalTrials.gov. Safety and Effectiveness Evaluation of the THERMOCOOL SMARTTOUCH SF Catheter with the TRUPULSE™ Generator for treatment of Paroxysmal Atrial Fibrillation (PAF) (SmartfIRE).
- Cleveland Clinic. Catheter Ablation. Accessed March 1, 2023
- Reddy VY, Neuzil P, Koruth JS, et al. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. JACC 2019;74(3)315-326.
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021 Feb;16(2):217-221. doi: 10.1177/1747493019897870. Epub 2020 Jan 19. Erratum in: Int J Stroke. 2020 Jan 28;:1747493020905964. PMID: 31955707.
- Global Burden of Disease Collaborative Network (2016) Global Burden of Disease Study 2016 (GBD 2016) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2017. Accessed 2018-04-20.
- Staerk, et al. 2018 Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. doi: 10.1136/bmj.k1453 | BMJ 2018;361:k1453
- Kuck et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23(3)362-369. PMID: 33330909
- ClinicalTrials.gov. A Study for Treatment of Paroxysmal Atrial Fibrillation (PAF) by Pulsed Field Ablation (PFA) System With Irreversible Electroporation (IRE) (inspIRE).


 January 29, 2026
January 29, 2026