July 14, 2023 — BIOTRONIK announced the one-year subgroup results from the investigator-initiated BIOPACT randomized controlled trial (RCT), which were presented by principal investigator Dr. Koen Deloose at LINC, the Leipzig Interventional Course 2023. The randomized controlled non-inferiority trial evaluated the safety and efficacy of the Passeo-18 Lux drug-coated balloon (DCB) catheter compared to the In.Pact Admiral DCB (Medtronic) and showed excellent results for both balloons through 12 months across a variety of sub-cohorts.
The prospective, multicenter, core-lab adjudicated non-inferiority study enrolled 302 patients in Austria, Belgium, France and Switzerland with Rutherford 2-4 disease.1 Patients were randomized 1:1 to either the Passeo-18 Lux DCB or In.Pact Admiral DCB for the treatment of stenotic, non-stented restenotic or occlusive lesions in the femoropopliteal artery.1
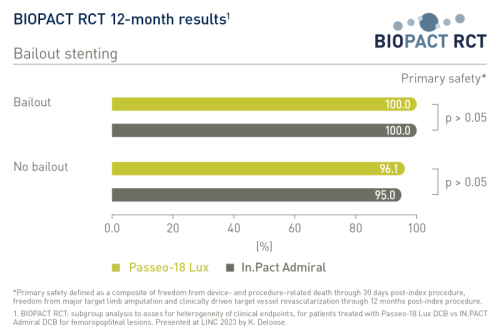
The primary safety endpoint was a composite of freedom from device- and procedure-related death through 30 days post-index procedure, freedom from major target limb amputation and clinically driven target vessel revascularization through 12 months post-index procedure. It showed no statistically significant difference between balloons for the full data set and across subgroups1:
- Diabetes: 94.7% vs 95.2% (Passeo-18 Lux DCB vs In.Pact Admiral DCB in diabetics); 97.1% vs 95.6% (Passeo-18 Lux DCB vs In.Pact Admiral DCB for non- diabetics)2
- Lesion length: 95.9% vs 90% (Passeo-18 Lux DCB vs In.Pact Admiral DCB, respectively, for patients with target lesions ≥ 100 mm); 96.7% vs 97.9% (Passeo-18 Lux DCB vs In.Pact Admiral DCB for patients with target lesions < 100 mm)2
- Calcium: 98.1% vs 96.8% (Passeo-18 Lux DCB vs In.Pact Admiral DCB, respectively, in severely calcified lesions); 92.9% vs 89.7% vs (Passeo-18 Lux DCB vs In.Pact Admiral DCB in none/moderately calcified lesions)2
- Bailout stenting: 100% vs 100% (Passeo-18 Lux DCB vs In.Pact Admiral DCB, respectively, with bailout stenting); 96.1% vs 95% (Passeo-18 Lux DCB vs In.Pact Admiral DCB without bailout stenting)2
- Occlusions: 100% vs 100% (Passeo-18 Lux DCB vs In.Pact Admiral DCB, respectively, in occlusions); 95.8% vs 94.7% (Passeo-18 Lux DCB vs In.Pact Admiral DCB in stenoses)2
“Interestingly, we found that across all variations and cohorts, there was no statistically significant difference in safety, primary patency, or clinically driven target lesion revascularization,” said Principal Investigator Dr. Koen Deloose, Head of the Department of Vascular Surgery, AZ Sint Blasius Hospital in Dendermonde, Belgium. “There is a scarcity of randomized head-to-head data, especially in the drug-coated balloon space, so the more data we have available, the more answers we have.”
One-year results from the full cohort, presented last year at Paris Vascular Insights 2022, showed comparable results between treatment arms, with a freedom from clinically driven target lesion revascularization rate of 97.2% for Passeo-18 Lux DCB and 97.0% for In.Pact Admiral DCB (P = 0.0002).1
The Passeo-18 Lux DCB has a paclitaxel dose of 3.0 µg/mm2 and uses a Butyryl-tri-hexyl citrate excipient (In.Pact Admiral DCB: 3.5 µg/mm2 and urea excipient). The lower-profile Passeo-18 Lux DCB is on an 0.018” platform and is 4F compatible for diameters of 2.0 to 4.0 mm and 5F compatible for diameters of 5.0 to 7.0 mm.3 In comparison, the In.Pact Admiral DCB is 5F compatible only for its 4.0 mm diameter, and is 6F or 7F compatible for its diameters of 5.0 to 7.0 mm.3,4
“We are thrilled to have these data for Passeo-18 Lux DCB,” said Stuart Perks, Vice President of Marketing, Vascular Intervention at BIOTRONIK. “While we have already seen Passeo-18 Lux DCB perform well across challenging patient groups in the BIOLUX P-III registry, it is great to now have a head-to-head RCT confirm these excellent safety and efficacy results.”
The BIOPACT study will continue collecting follow-up data at 24, 36, 48 and 60 months, which will be shared upon completion of each follow-up period. The 24-month full-cohort results will be announced later this year.
References:
1 Deloose K. BIOPACT RCT: a third head-to-head DCB RCT. Presented at: Paris Vascular Insights; November 2022; Paris, France.
2 Deloose K. BIOPACT RCT: subgroup analysis to asses for heterogeneity of clinical endpoints, for patients treated with Passeo-18 Lux DCB vs IN.PACT Admiral DCB for femoropopliteal lesions. Presented at: LINC; June 2023; Leipzig, Germany.
3 BIOTRONIK data on file.
4 Competitor matrix and specification taken from Endovascular Today. European device guide: drug-coated balloons Accessed June 2023. https://evtoday.com/device-guide/european/drug-coated-balloons-1
Passeo-18 Lux paclitaxel-releasing PTA balloon catheter is not available for sale or use in the United States.
Passeo and Lux are trademarks or registered trademarks of the BIOTRONIK Group of Companies. All other trademarks or registered trademarks are the property of their respective owners.
For more information: BIOTRONIK Passeo 18 Lux - Drug-Coated Balloon Catheter


 January 05, 2026
January 05, 2026 









