November 17, 2023 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, announced the Food and Drug Administration (FDA) approval of its Phase III clinical trial of its CardiAMP autologous cell therapy for the treatment of patients with ischemic heart failure.
BioCardia announces that the Food and Drug Administration (FDA) has approved its proposed CardiAMP Heart Failure II study protocol. The currently ongoing CardiAMP Heart Failure trial has completed enrollment and it is anticipated that the final data analyses will be reported in Q4 2024. In an interim analysis of available data to date for study patients followed up through two years, those having N-terminal pro B-type natriuretic peptide (NT-proBNP) levels consistent as demarcating heart failure (>500 pg/ml) at screening-baseline showed meaningful clinical improvements over controls, including a 59% relative risk reduction in heart death and a 54% relative risk reduction of Major Adverse Cardiovascular or Cerebrovascular events (MACCE). Further, all clinical outcome measures evaluated at follow-up for this interim subset favored cell therapy over guideline directed medical therapy, including having improved quality-of-life as measured using the Minnesota Living with Heart Failure Questionnaire, reduced NT-proBNP levels, greater walk distance as measured using the 6-minute walk distance test, and improved cardiac measures such as left ventricular ejection fraction and left ventricular end systolic and end diastolic volumes. Statistical significance (p<0.05) was noted for both the reduced heart death equivalents measure (p=0.028) and the improved quality of life measure (p=0.016).
The FDA has approved the proposed CardiAMP Heart Failure II study which includes an eligibility requirement that patients demonstrate a pre-specified NT-proBNP level at baseline. The proposed primary efficacy endpoint is also modified from that in the currently ongoing study. The CardiAMP Heart Failure II endpoint is a similar hierarchical composite assessment but consists of all-cause death, the cardiac death equivalents of heart transplant and left ventricular assist device (LVAD) implantation, heart failure hospitalizations, worsening heart failure events treated as an outpatient, and change in quality-of-life, with a follow-up duration ranging from a minimum of 12 to a maximum of 24 months. Statistical power calculations using the interim data analyses support a modestly sized clinical trial would demonstrate efficacy if the interim data it is based on is representative of future study patients enrolled into the trial. Additional modifications from the ongoing trial include enhancements to simplify clinical site logistics and reduce costs of the study. Medicare’s reimbursement support for the trial currently in place for both the control and treatment arms is anticipated to significantly offset clinical costs of the trial.
“CardiAMP Heart Failure II has strong support from our clinical investigators who have reviewed the interim trial results in detail and have first-hand knowledge of this great unmet clinical need,” said Peter Altman, PhD., BioCardia’s President and Chief Executive Officer. “We expect that we can perform this study efficiently at world class clinical centers based on the extensive experience of the ongoing trial and its positive clinical outcomes.”
About the CardiAMP Cell Therapy Program
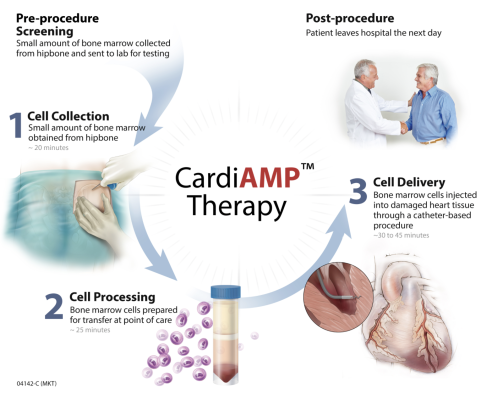
CardiAMP Cell Therapy – FDA designated as a Breakthrough therapy – uses a patient’s own (autologous) bone marrow cells delivered to the heart in a minimally invasive, catheter-based procedure to potentially stimulate the body’s natural healing response. The CardiAMP Heart Failure II Trial builds on positive clinical experience with this autologous cell therapy in almost 200 patients and has potential to provide the primary evidence to support FDA approval and marketing registration. The trial is supported by the Maryland Stem Cell Research Fund and the Centers for Medicare and Medicaid Services. CAUTION - Limited by United States law to investigational use.
For more information: www.biocardia.com


 February 03, 2026
February 03, 2026 









