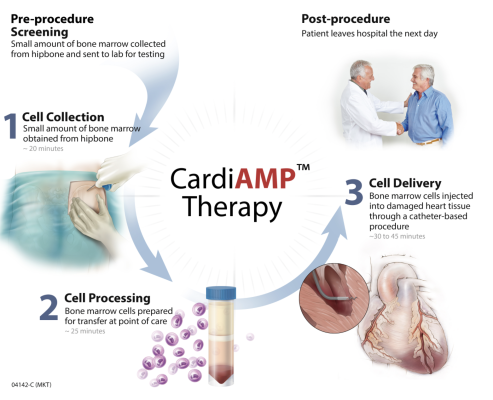
September 8, 2023 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary disease, announced a clarification and next steps on its autologous CardiAMP cell therapy programs.
Based on the recent interim results in the CardiAMP autologous cell therapy for the treatment of heart failure (BCDA-01), the Company is exploring development of a new Phase III clinical trial protocol. The Finkelstein Schoenfeld (FS) primary composite endpoint used in the CardiAMP HF Trial has tiers of outcomes in decreasing order of importance: heart death equivalent, major adverse cardiac and cerebrovascular MACCE, and Six Minute Walk Distance (6MWD). Since the primary FS endpoint is a composite of these elements, meeting the FS endpoint may be met even if one or more of the components does not individually demonstrate statistical significance. This has implications for the future trial design.
The CardiAMP HF Trial interim results showed that the first two most important tier outcomes, occurring in 30% of the study patients, could have contributed sufficiently toward efficacy given a longer follow-up period than one year. If these interim results are replicated in a future study in which the third tier 6MWD is replaced with a more objective endpoint, there may be a pathway to a successful trial for product registration in the United States. The FDA has previously expressed a preference for replacing the 6MWD with Cardiopulmonary Exercise Testing, which is a more objective outcome. We are exploring with the CardiAMP HF Study Executive Steering Committee whether to fine tune eligibility criteria for patients based on current data and to replace the 6MWD with Cardiopulmonary Exercise Testing or another other more objective third-tier outcome measurement.
Such a protocol may also be capital efficient by eliminating measures that have already been gathered in the ongoing CardiAMP HF study while continuing to utilize the CMS reimbursement program currently in place. Using the cell population analysis screening criterion to set patient dosage, rather than using it to exclude patients from the study, could accelerate enrollment.
CardiAMP in Chronic Myocardial Ischemia, or BCDA-02, has a generally accepted mechanism of action of microvascular revascularization driven by the CD34 and CD133 components of the dosage. It is a different indication from the heart failure indication and members of the Steering Committee believe it has great potential to be successful even if the heart failure indication is not ultimately successful. Microvascular revascularization and repair to reduce pain and enhance heart performance in a relatively healthy heart is viewed as an easier challenge for the cells to overcome than helping a heart already in failure to recover. This study is on track to complete the roll in cohort enrollment in Q4 and advance to its randomized double blind pivotal trial. BioCardia expects to focus its resources on accelerating this pivotal program ahead and will be incorporating strategies to enhance enrollment.
“We have three synergistic clinical programs for the treatment of ischemic heart disease utilizing the leading transendocardial delivery platform which we also developed,” continued Peter Altman. “Our expectation is that we can move all of these programs through significant milestones in the next year on less capital than we have utilized in previous years.”
Anticipated Upcoming Milestones and Events:
- BCDA-01: CardiAMP Cell Therapy for Heart Failure Phase III Trial
- Q3 2023: Clarity on Second Pivotal Study Design
- Q4 2023: Japan PMDA Formal Consultation
- BCDA-02: CardiAMP Cell Therapy for Chronic Myocardial Ischemia Phase III Trial
- Q4 2023: Completion of Roll-in Cohort and Transition to Randomized Pivotal Trial
- BCDA-03: NK1R+ MSC Allogeneic Cell Therapy in ischemic HFrEF Phase I/II Trial
- Q3 2023: First Patient Enrolled
- Helix Biotherapeutic Delivery System
- Q4 2023: Completion of Enrollment in Partner CellProthera’s EXCELLENT Trial
- Q4 2023: Update on Licensing / Partnerships
For more information: www.biocardia.com


 January 05, 2026
January 05, 2026 









