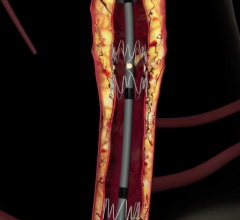
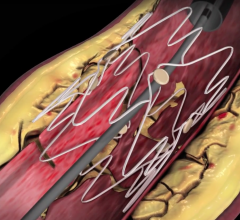
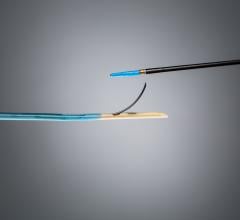
Assurant Cobalt Iliac Balloon-Expandable Stent System
Related Content
November 6, 2019 – The final, long-term, patient-level data for the Cook Medical Zilver PTX drug-eluting stent (DES) ...
October 5, 2018 — Veryan Medical Ltd has received Premarket Approval (PMA) for the BioMimics 3D Vascular Stent System ...
April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is ...
July 26, 2017 — Intact Vascular Inc. recently announced that its Tack Optimized Balloon Angioplasty II Below the Knee ...
July 14, 2017 — Intact Vascular Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational ...
April 28, 2017 — Biotronik’s Pulsar-18 bare metal stent (BMS) has yielded high primary patency in a real-world setting ...
March 16, 2017 — W. L. Gore & Associates Inc. recently announced the Health Canada approval of the Gore Tigris Vascular ...
March 2, 2017 — Intact Vascular Inc. announced in February that its Tack Optimized Balloon Angioplasty II Below the Knee ...
December 13, 2016 — Biotronik announced the presentation of data confirming the efficacy of the Pulsar-18 bare metal ...
December 8, 2016 — LimFlow SA announced in November that it received the CE Mark for its fully percutaneous LimFlow ...


 November 06, 2019
November 06, 2019