December 7, 2023 — Ajax Health announced today the formation and funding of Cortex, a medical technology company developing an integrated mapping and ablation solution suite for the treatment of atrial fibrillation (AFib), the most common heart rhythm disorder, affecting more than 30 million patients worldwide. Cortex has raised $90 million in funding commitments led by KKR and Hellman & Friedman (H&F) with participation by other investors including AI Life Sciences, an affiliate of Access Industries.
Cortex brings together expert teams with complementary innovations in electrophysiology to accelerate the continued development and clinical validation of next-generation ablation solutions and differentiated AFib mapping technology. The fully integrated solution suite is designed to enable more precise therapy planning and delivery and optimize clinical outcomes and safety for AFib patients, while simplifying workflows and improving procedural efficiency.
"Cortex's vision is to enable more intelligent AFib treatment," said Duke Rohlen, CEO of Ajax Health and CEO of Cortex. "We are developing solutions that prioritize precision, simplicity, and efficiency to simultaneously improve patient outcomes and lower procedural cost."
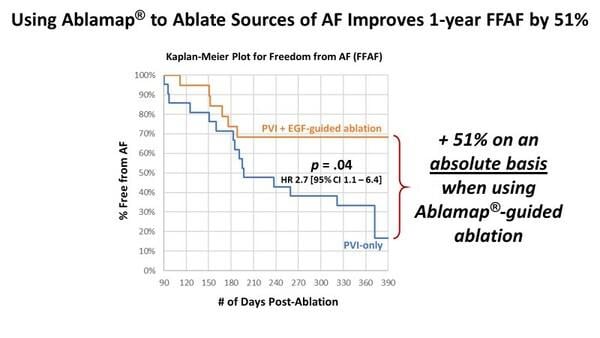
Cortex is focused on developing diagnostic and pulsed field ablation catheters with a comprehensive mapping and navigation solution powered by Ablacon's innovative Electrographic Flow® (EGF) mapping technology. EGF mapping allows physicians to detect AFib sources and is designed to support personalized ablation therapy to potentially improve outcomes. The recently completed randomized, controlled FLOW-AF trial (NCT04473963) showed that EGF-guided treatment of AFib sources in persistent AFib patients improved freedom from AFib at one year post-ablation by 51% on an absolute basis compared with patients randomized to control, who received conventional pulmonary vein isolation therapy only. Ablacon's latest Ablamap X mapping system is 510(k) cleared. Following on the favorable results of FLOW-AF, the company has launched the RESOLVE-AF trial (NCT05883631), a large, international, multi-center clinical trial to further evaluate benefits in AFib patients and support CE mark application.
“Duke Rohlen and the Ajax team have cultivated an exceptional ecosystem of engineers and clinical experts with a clear plan to bring impactful new technology to clinical settings,” said Ali Satvat, Partner, Co-Head of Americas Health Care and Global Head of Health Care Strategic Growth at KKR. “We are pleased to continue our long-standing strategic partnership with Duke and join with a strong investor group to support Cortex as it pursues improved outcomes for cardiac arrhythmia patients.”
KKR and H&F are investing in Cortex through the Cordis Accelerator, Cordis-X, which was established in 2021 as part of their investment in Cordis, a leading provider of cardiovascular and endovascular medical devices. KKR is investing additional capital in Cortex through its Health Care Strategic Growth Fund II.
For more information: https://www.ajaxhealth.com/


 November 14, 2025
November 14, 2025 









