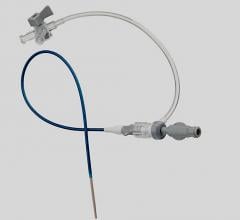
December 11, 2008 - Access Scientific Inc. said this week it received FDA clearance for its MicroAccess WAND, which the company said is the world’s first all-in-one safety introducer.
This new medical device enables clinicians to more quickly and safely insert a sheath or catheter into the peripheral vasculature, the company said. The MicroAccess WAND is the first of several planned WAND devices. The devices combine all components of the older, modified Seldinger technique into a unitary device that provides faster, safer, simpler over-wire vascular access.
The MicroAccess WAND is expected to be used primarily in interventional radiology suites and cardiac catheterization labs.
In addition to uniting the elements needed to perform the Seldinger technique into one unitary device, The WAND is designed to reduce the risk of accidental needle sticks, bleeding, contamination, guide wire embolism, and loss of cannulation. Its Fast-flash feature provides early detection of vessel entry.
For more information: www.The-Wand.com


 June 10, 2020
June 10, 2020