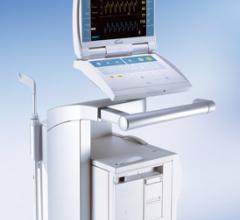
July 13, 2011 – The TandemHeart circulatory support system has been granted expanded reimbursement coverage by NHIC Corp ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Maquet Datascope Corp. has issued a class I recall for ...
June 14, 2011 – An initial animal study of a next-generation cardiac assist device for heart failure patients using a ...
May 11, 2011 – The Impella percutaneously deployed ventricular assist device showed an overall average hospital charge ...
May 3, 2011 – The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to Maquet Cardiovascular’s ...
April 19, 2011 – Promising new devices for mechanical circulatory support of children with heart defects or heart ...
April 13, 2011 – The American College of Cardiology Foundation (ACCF), American Heart Association (AHA) and the ...
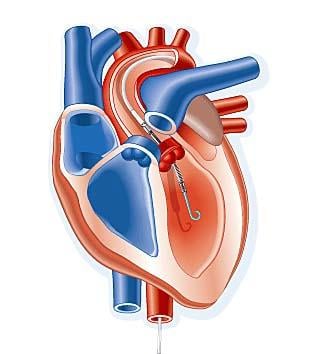
April 8, 2011 — A transcatheter ventricular assist device performed better than intra-aortic balloon pumps (IABP), with ...
March 28, 2011 – The Therapeutic Goods Administration (TGA) of Australia has approved the HeartWare Ventricular ...
March 11, 2011 – Texas Children's has become the world's first pediatric hospital to complete the first phase of ...
February 17, 2011 – On Jan. 15, after 864 days of life with an artificial heart, Charles Okeke received a dual ...
January 27, 2011 – The U.S. Food and Drug Administration (FDA) has approved an Investigational Device Exemption ...
January 26, 2011 - The University of Michigan Cardiovascular Center and the University of Pittsburgh have been ...
January 13, 2011 – HeartWare International has submitted a Pre-Market Approval (PMA) application to the U.S. Food ...


 July 13, 2011
July 13, 2011