June 13, 2018 — Henry Ford Hospital is one of 17 U.S. trial sites using a catheter-based procedure approved in Europe to ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
June 7, 2018 — The first clinical cases have been completed where the Emboliner Embolic Protection Catheter was used ...
June 1, 2018 – Use of the Abbott Portico transcatheter aortic valve replacement (TAVR) therapy was associated with ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

Here are the late-breaking trials and other key study presentations from the 2018 EuroPCR conference. This is the annual ...
May 25, 2018 — Abbott announced favorable outcomes from the first 100 patients treated in a global study of its Tendyne ...
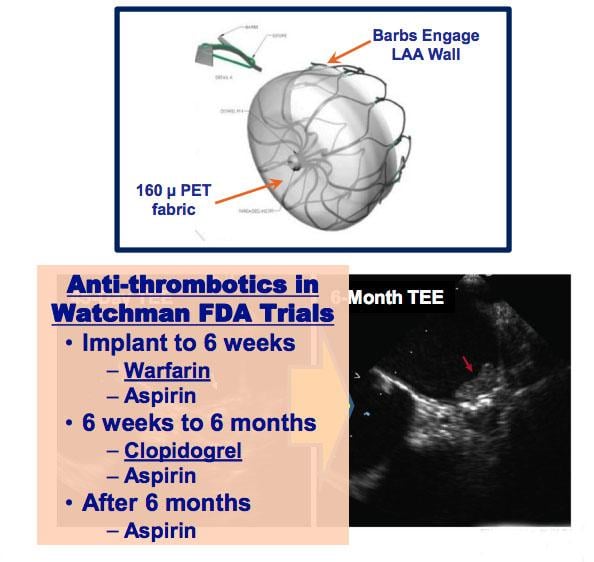
May 18, 2018 — Left atrial appendage closure (LAAC) with the transcatheter Watchman device prevents thromboembolism from ...

May 14, 2018 – A new study finds patients who stay in the hospital for more than 72 hours when undergoing trans-femoral ...
May 14, 2018 – A new study examines the effectiveness of 3-D printing technology and computer modeling to predict ...
May 14, 2018 — The opioid drug epidemic is impacting cardiology, with a new study finding the number of patients ...
May 14, 2018 — Ancora Heart Inc. announced the expansion of the company’s U.S. feasibility study to evaluate the ...
May 11, 2018 — A new study looked at the effectiveness of a novel risk tool to predict 30-day readmission rates in ...
May 11, 2018 — Medtronic plc announced two-year outcomes for the Harmony Transcatheter Pulmonary Valve (TPV) from its ea ...
May 11, 2018 — The post-approval study evaluating the safety and efficacy of MitraClip in a real-world, commercial ...
May 8, 2018 — One-year results from the Sentinel Cerebral Protection System show it can reduce the incidence of stroke d ...
May 7, 2018 — Colibri Heart Valve LLC announced the Colibri transcatheter aortic valve implantation (TAVI) System has ...


 June 13, 2018
June 13, 2018










