My observation over the 13 years covering cardiology is that it is a heavily male-dominated field. This is more ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
The latest in interventional cardiology clinical data and new device technologies were highlighted at the annual Transca ...

November 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) m ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
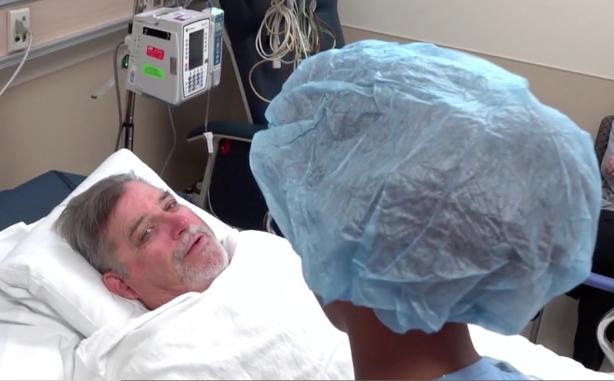
October 29, 2019 — When Scripps cardiologists discovered early in 2019 that retired National Football League (NFL) great ...
October 28, 2019 — The Gore Cardioform ASD Occluder received European CE mark clearance in early October. The device ...
Ashish Pershad, M.D., chief of interventional cardiology, Banner University Medical Center, Phoenix, explains the trend ...
Vivian Ng, M.D., assistant professor of medicine and an interventional cardiologist at the NewYork-Presbyterian/Columbia ...

October 10, 2019 — Here are the Cardiovascular Research Foundation (CRF) 12 late-breaking trials and 16 late-breaking ...
Torsten Vahl, M.D., director of experimental and translational research, Structural Heart and Valve Center and at the ...
Chandan Devireddy, M.D., offers insights about what he saw as the top take aways from the 2019 Transcatheter ...
October 4, 2019 – A new analysis of the PARTNER 3 Trial data found a modest, but significant, improvement in one-year ...
October 4, 2019 – Results of a new economic analysis of the COAPT Trial data found that transcatheter mitral valve ...
October 3, 2019 – Five-year results from the PARTNER 2A Trial found patients with severe aortic stenosis (AS) and ...
October 3, 2019 – The three-year results from the COAPT Trial demonstrated that reducing severe secondary mitral ...
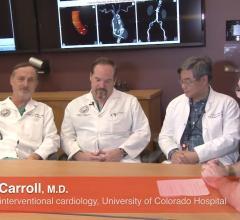
Interview with John Carroll, M.D., director of interventional cardiology, Robert Quaife, M.D., director of advanced ...


 November 04, 2019
November 04, 2019