A study from a South Korean research team found that angioplasty with a sirolimus-eluting stent was non-inferior to ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.

April 6, 2011 – One-year results from the pivotal PLATINUM Workhorse trial were presented at the American College ...
April 6, 2011 – Results from a pooled patient-level analysis favored a next-generation paclitaxel-eluting stent ...
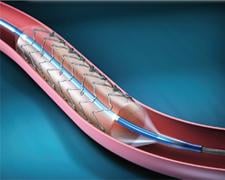
April 6, 2011 – A pooled analysis of the SPIRIT II, III, IV and COMPARE trials further reinforces the positive ...
March 21, 2011 - OrbusNeich today announced it filed a lawsuit in the Netherlands against a doctor and principal ...
February 21, 2011 – Two drug-eluting coronary stent systems have been launched in India. Boston Scientific’s ...
February 21, 2011 – The first patient has been enrolled the DESSOLVE II study to support CE mark for a coronary ...
January 26, 2011 – Patient enrollment has begun in China for a trial evaluating the safety and effectiveness of a ...
January 24, 2011 – Patient enrollment was completed in the EVOLVE clinical trial, which is designed to assess the ...
January 20, 2011 – European CE mark approval was granted to expand the indication of Abbott’s Xience Prime ...
January 20, 2011 – Drug-coated stents hold promise as a safe and lasting solution for the treatment of clogged leg ...
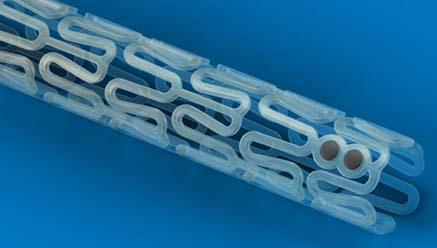
January 10, 2011 – European CE mark approval was announced today for the Abbott, Absorb drug-eluting ...
December 6, 2010 - A European court has struck a blow to Boston Scientific's alleged patent infringement case ...
September 28, 2010 – Three-year follow-up data from the HORIZONS-AMI trial presented at Transcatheter ...
September 24, 2010 – Drug-eluting stents are beneficial in treating symptomatic peripheral artery disease (PAD) in ...


 April 08, 2011
April 08, 2011








