March 20, 2020 — The Centers for Medicare and Medicaid Services (CMS) announced March 18, 2020, that all elective ...
Heart Failure
This heart failure channel offers news and new technology to treat heart failure. This includes for new innovations to treat congestive heart failure (CHF). The channel includes news on HFpEF and HFrEF. Heart failure occurs when the heart is no longer able to pump as much blood as the body requires. This can lead to enlargement of the heart because the muscle works harder to supply blood, but the pumping is ineffective. This may be due to defects in the myocardium, such as an infarct, or due to structural issues such as severe valve regurgitation. The disease is divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or a left ventricular assist device (LVAD).
Interview with Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and ...
March 10, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the SynCardia Systems 50cc temporary Total ...
New research shows superiority of Ultromics’ AI in predicting cardiac-related death As the use of artificial ...
March 5, 2020 — Abbott recently received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA ...
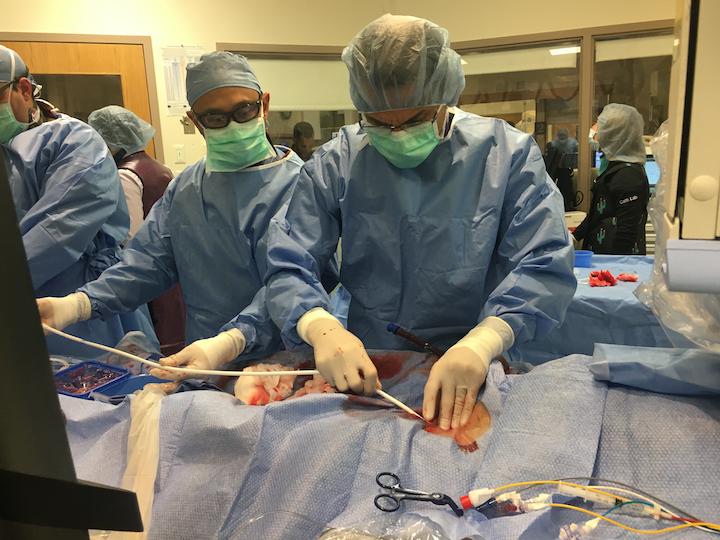
Lara Reyelt, veterinary technician and preclinical surgeon at the Interventional Research Laboratories (SIRL) at Tufts ...

There are several new tools being added to the clinical armamentarium in the fight against heart failure (HF). These ...
There is no, single magic bullet in heart failure (HF) to easily reduce readmission rates or easily reverse this complex ...
February 12, 2020 — The U.S. Food and Drug Administration (FDA) has approved Carmat's investigational device exemption ...
Interview with James Udelson, M.D., chief of the division of cardiology at Tufts Medical Center, Boston. The hospital ...
January 22, 2020 – BioVentrix Inc., developer of the first transcatheter device for left ventricular remodeling after a ...
January 9, 2020 – Researchers may have discovered a way to turn back the clock on aging heart muscles in fruit flies, a ...
January 7, 2020 — Abbott announced U.S. Food and Drug Administration (FDA) approval of a new alternative surgical ...
January 7, 2020 — The U.S. Food and Drug Administration (FDA) has accepted a supplemental new drug application (sNDA) ...
December 19, 2019 — The U.S. Food and Drug Administration (FDA) has granted breakthrough status for a novel ECG-based ...
December 18, 2019 — BioVentrix, Inc., developer of the first less invasive system for left ventricular remodeling, today ...

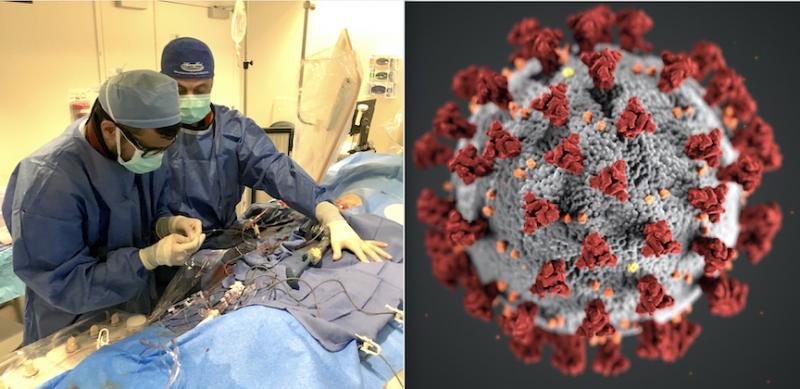
 March 20, 2020
March 20, 2020