July 13, 2010 – AstraZeneca recently announced that the U.S. District Court has found that the substance patent protecting Crestor is valid and enforceable. In its ruling, the court found that no inequitable conduct was committed by any Shionogi employee. The court also held the '314 patent to be non-obvious and properly reissued.
July 13, 2010 – A new meta-analysis of five implantable cardioverter-defibrillator (ICD) studies shows a smaller impact of sudden cardiac death (SCD) on overall mortality in women.
July 12, 2010 – AngioDynamics announced today the global expansion of its Smart Port CT family of power-injectable ports, featuring its patented Vortex port technology, to include low-profile and mini models for repeated treatments such as chemotherapy and for use with computed tomography (CT).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 12, 2010 – GE Healthcare Medical Diagnostics today announced the market reintroduction of Optison (Perflutren Protein-Type A Microspheres Injectable Suspension, USP), a diagnostic ultrasound contrast agent for use in select echocardiograms.
July 12, 2010 – Lantheus Medical Imaging Inc. today announced that it has acquired the balance of the worldwide rights for gadofosveset trisodium, a unique, injectable magnetic resonance angiography (MRA) blood pool imaging agent that it currently markets in the United States as Ablavar.
July 12, 2010 – Health Level Seven Intl. (HL7), a non-profit standards development organization, has submitted comments emphasizing the need for strong health informatics standards to the European Union’s recent public open consultation on the review of the European Standardization System.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 9, 2010 – Lantheus Medical Imaging Inc. announced this week that it has signed a new manufacturing and supply agreement with MDS Nordion to purchase molybdenum-99 (Mo-99), the parent isotope of technetium-99m (Tc-99m).
July 9, 2010 – W. L. Gore and Associates announced today that they are confident in the design and anticipated results of the Gore REDUCE Clinical Study, which was designed to test the safety and effectiveness of the Gore Helex Septal Occluder for patent foramen ovale (PFO) closure in patients with history of cryptogenic stroke or imaging-confirmed transient ischemic attack (TIA).

Single photon emission computed tomography (SPECT) nuclear imaging and computed tomography (CT) scanners have been used separately for cardiology, oncology and neurologic imaging for more than 20 years. In recent years there has been a trend toward combined SPECT/CT systems to enable faster and more accurate functional and anatomic assessment using a single imaging system in one imaging session.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 8, 2010 – The annual Transcatheter Cardiovascular Therapeutics (TCT) event has traditionally been a symposium for interventional cardiologists. However, as an increasing number of hybrid intervention/minimally invasive surgical procedures bring surgeons into the arena of cath lab techniques, TCT started a course for surgeons who specialize in hybrid procedures.
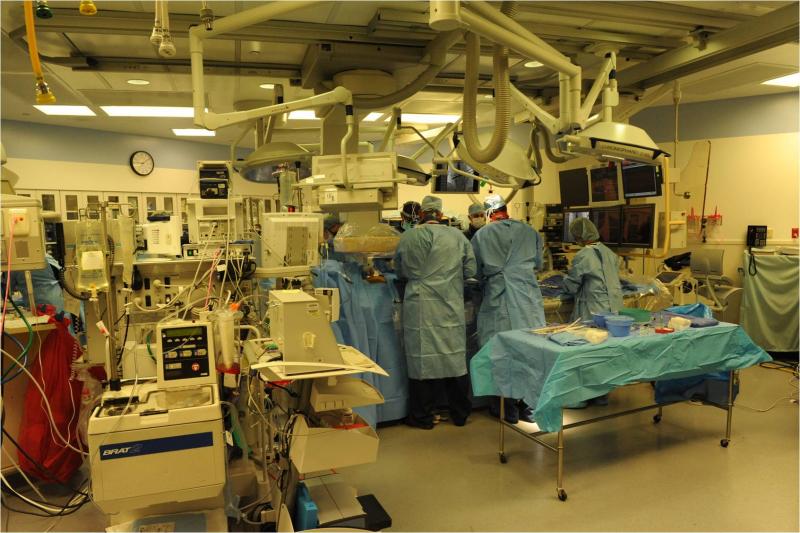
The barriers that once separated interven-tionalists, cardiac surgeons, vascular surgeons and electrophysiologists are rapidly being reduced by the convergence of transcatheter technologies and the need for high-quality fluoroscopic imaging systems.
July 8, 2010 – Users of eMix’s cloud-based sharing technology now have the ability to preview exams from any Web browser or smart phone, thanks to a new feature called eMix Preview. Any authenticated users can now preview the reports and images of any eMix exam package and save them directly into their electronic medical record (EMR).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
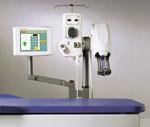
Automated contrast media injectors are used in cardiac imaging to help improve patient safety and enhance image quality. These devices control contrast dosage, record the amount used, speed injections to keep up with fast CT scanners, and warn of potential hazards, such as air embolisms or extravasations. Key Differences in Injectors
July 8, 2010 – Siemens Healthcare recently announced the 2.0 release of its Acuson S2000 ultrasound system, with applications across general imaging, including obstetrics and gynecology (OB/GYN), as well as vascular and cardiac imaging. The new release features: • Next generation tissue strain analytics, including Virtual Touch HD technology and
July 8, 2010 – A new consensus statement released recently by the Heart Rhythm Society (HRS) offers guidance for managing cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting device deactivation.

 July 13, 2010
July 13, 2010











