November 21, 2011: Each year, more than 600,000 Americans are stricken with a pulmonary embolism (PE), which is a clot in the lung. Without immediate help, almost half of these individuals may develop pulmonary hypertension, or worse, die. More Americans die each year from a PE than from AIDS, breast cancer and motor vehicle accidents combined. The current method of treatment has been high doses (100 mg) of a clot-dissolving drug administered intravenously over a two-hour period, or the use of anticoagulants. Unfortunately, high doses of clot-dissolving drugs have shown to cause bleeding, the worst of which is a hemorrhage in the brain. Anticoagulants help prevent more clot build up, but do not dissolve the blood clot. If your body does not dissolve the clot fast enough, the right side of the heart becomes enlarged trying to push blood past the clot and heart failure could occur.
November 21, 2011 - Frank J. Veith, M.D., founder and chairman of the VEITH symposium, the William J. von Liebig Chair in Vascular Surgery, Cleveland Clinic and New York University Medical Center, took a strong position against the current guidelines of the American Heart Association (AHA) that support the use of carotid artery stenting (CAS), as opposed to the more traditional open carotid endarterectomy (CEA), to treat symptomatic carotid stenosis (CS) in low to moderate risk patients. Veith explained to an audience of his peers that an important clinical trial for CAS, the CREST (Carotid Revascularization Endarterectomy vs. Stent Trial), had results that appeared to show that the stent procedure (CAS) and the open surgical approach (CEA) were equivalent. Veith argued that the study was in fact flawed in several ways and that the AHA guideline was misguided.
November 17, 2011 — Westside Medical Imaging of Beverly Hills announces the availability of high resolution magnetic resonance imaging (MRI) with a 3 Tesla magnet to monitor the effects of cholesterol drugs on atherosclerosis.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 18, 2011 – Boston Scientific’s Watchman left atrial appendage (LAA) closure device has been implanted in the first patients in Latin America. The device is designed for use in patients in atrial fibrillation who are at risk for stroke and are eligible for long-term oral anticoagulation therapy such as warfarin. Watchman is intended to prevent embolization of thrombi that may form in the LAA, thereby preventing the occurrence of ischemic stroke and systemic thromboembolism in patients with non-valvular atrial fibrillation. The first patient implants were performed by Bernardo Caicedo, M.D., interventional cardiologist, at Angiografia de Occidente in Cali, Colombia.
November 18, 2011 – W. L. Gore & Associates Inc. announced that it has received approval from the U.S. Food and Drug Administration (FDA) to market the Conformable Gore Tag thoracic endoprosthesis as a minimally invasive treatment for patients suffering from thoracic aortic aneurysms (TAAs). The announcement came at the VEITH Symposium 2011 Conference in New York. The device is the only FDA-approved polytetrafluoroethylene (PTFE) thoracic endoprosthesis designed for endovascular repair of the descending thoracic aorta that offers conformability and ease of use, while accommodating tapered anatomy and resisting compression. The broad oversizing window for the device ranges from six to 33 percent, allowing physicians to choose the appropriate oversizing for the patient anatomy.
November 18, 2011 – The first comprehensive report to review timing of treatment for patients with blockages in more than one coronary artery recommends a flexible approach that meets individual patient’s needs. The report was issued today by the Society for Cardiovascular Angiography and Interventions (SCAI) and is published online in Catheterization and Cardiovascular Interventions.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
November 18, 2011 —The Berlin Heart Group announced today that clinical trial data were presented this morning at the “Scientific Sessions 2011” annual meeting of the American Heart Association (AHA). These data showed that, in a clinical trial of children with severe heart failure who were waiting for a heart transplant, 90 percent of patients in the primary cohort of the study were successfully bridged to a heart transplant using Berlin Heart Excor pediatric ventricular assist device (VAD).
November 18, 2011 – Stroke remains a major healthcare problem; it currently ranks as the third most common cause of death in Western nations, and this is only expected to increase as the population ages. For this reason, early identification of symptoms that are known predictors of stroke is a topic that was addressed with great deliberation and discussion at the 2011 VEITH symposium.
November 18, 2011 – MedSolutions, a provider of medical cost management services, announced the launch of its implantable cardioverter defibrillator (ICD) surgery management program, which uses evidence-based guidelines to ensure the clinical appropriateness of ICD and CRT-D (cardiac resynchronization therapy defibrillator) implantation and directs members to the most qualified physicians and facilities.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 18, 2011 – An analysis of the TRITON-TIMI 38 trial presented today in an oral session at the American Heart Association (AHA) Scientific Sessions meeting examined the effect of Effient (prasugrel) on cardiovascular (CV) events (CV death, myocardial infarction [MI] and stroke) compared to clopidogrel relative to the timing of percutaneous coronary intervention (PCI) in acute coronary syndrome (ACS) patients with ST-segment elevation myocardial infarction (STEMI).
November 18, 2011 – CorMatrix Cardiovascular Inc. announced that the U.S. Food and Drug Administration (FDA) has granted the company full investigational device exemption (IDE) approval for its ongoing multi-center, prospective, randomized clinical trial aiming to demonstrate the safety and efficacy of the CorMatrix ECM for Pericardial Closure to reduce the incidence of new onset postoperative atrial fibrillation following isolated primary coronary artery bypass graft (CABG) surgery. CorMatrix Cardiovascular is a medical device company dedicated to developing and delivering unique extracellular matrix (ECM) biomaterial devices that harness the body's innate ability to repair damaged cardiovascular tissue.
November 17, 2011 - Medical Simulation Corp. (MSC) launched an easy-to-use, portable, high-fidelity endovascular procedural simulator at TCT 2011. The Compass simulator is small enough to fit in one shipping case and can be checked as luggage on an airplane. It takes less than five minutes to set up and can be easily used in the physician's office.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medtronic and Bain Capital, a global private investment firm, today announced they have entered into a definitive agreement under which affiliates of Bain Capital will acquire Physio-Control. The stock of Physio-Control and related entities will be purchased for cash in a transaction valued at approximately $487 million.
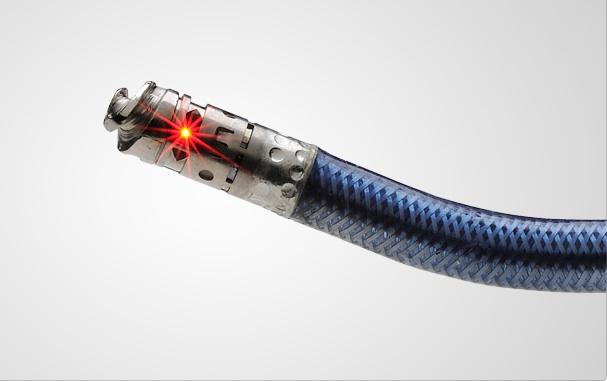
Each year I attend Transcatheter Cardiovascular Therapeutics (TCT), I comb the event and its sessions looking for the next big trend or technological innovations in cardiovascular devices. Below are my finds for the most cutting-edge and futuristic technologies at TCT 2011.
November 16, 2011 — Non-adherence to antiplatelet therapy – which prevents blood clots following percutaneous coronary intervention (PCI) – was associated with higher rates of both ischemic and bleeding events at 30 days. Results of the PARIS registry were presented at the 23rd annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, sponsored by the Cardiovascular Research Foundation.

 November 21, 2011
November 21, 2011










