Addressing concerns over radiation from cardiac imaging and procedures, the American College of Cardiology Foundation (ACCF), Duke University Clinical Research Institute (DCRI) and American Heart Association (AHA) released recommendations to enhance radiation safety.

March 20, 2012 – The Society for Cardiovascular Angiography and Interventions (SCAI) published a first-of-its-kind paper defining best practices in the cardiac catheterization laboratory, commonly referred to as the cath lab. The paper is part of SCAI’s ongoing effort aimed to ensure the highest quality care and safety for patients undergoing interventional cardiology procedures such as angioplasty and stenting (also known as percutaneous coronary intervention or PCI). Titled “Clinical Expert Consensus Statement on Best Practices in the Cardiac Catheterization Laboratory,” the paper is e-published in Catheterization and Cardiovascular Interventions.
March 20, 2012 - MarBlue Medical announced the global product launch of its CE-marked drug-eluting balloon (DEB) Protege as well as its CoCr stent on DEB Pioneer. The full market launch of the new DEB technology is a major step forward in Blue Medical's quest to improve the quality of life of patients with cardiovascular diseases, while at the same time reducing procedure costs.
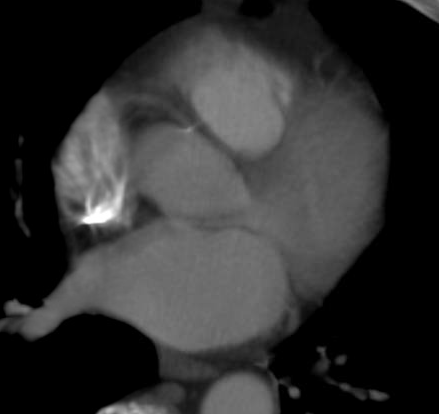
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 20, 2012 - GE Healthcare is demonstrating its new Centricity Cardio Enterprise solution during the American College of Cardiology (ACC) 2012 meeting in Chicago. The integrated cardiovascular clinical informatics system includes both CVIS (cardiovascular information system) and CPACS (cardiovascular picture archiving and communication system) functionality to provide clinicians full access to a single, comprehensive cardiovascular patient record. Centricity Cardio Enterprise features Web-enabled technology capable of driving workflow efficiencies and integrating with your current IT systems.
March 20, 2012 - DR Systems announced new contracts with four facilities for the company's Unity CVIS (Cardiovascular Information System). The facilities are: Twin Cities Community Hospital, Good Shepherd Medical Center, HealthCare Partners Medical Group and St. Luke's Cornwall Hospital.

Jack Miller, 62, is probably the only politician in America who smiles when people call him “heartless.” Four months ago, doctors at Virginia Commonwealth University (VCU) Medical Center in Richmond removed his dying heart and replaced it with the SynCardia temporary Total Artificial Heart.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 19, 2012 - Cameron Health Inc. announced today that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel will review the premarket approval (PMA) application of the S-ICD System on April 26, 2012.
There has been a convergence of expertise, deciding what is the best approach for patients and combining skill sets, imaging systems and tools of both interventionalists and surgeons, is the recent trend of hybrid ORs.

Management of coronary chronic total occlusion (CTO) is individualized depending on the severity of symptoms, ischemia and on the severity of concomitant coronary artery disease (CAD). Treatment should include anti-anginal and other therapies to promote vascular health. Patients who remain symptomatic or have a large burden of ischemia despite maximal medical therapy can be considered for revascularization.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Cardiology departments have hosted a variety of software solutions in the past to meet varying demands. A recent report from KLAS reveals that the cardiology IT market is moving toward consolidation. The report, “Cardiology 2012: Will the Complete Cardiovascular Information System (CVIS) Please Stand Up?” explains a result of this consolidation trend is that providers are looking for a technology leader to step up and meet their needs.

Computer-aided detection (CAD) software constitutes a set of algorithms which, using a pattern-recognition technique, aids the radiologist in detecting potentially diseased regions and lesions. It uses clinical images obtained from various imaging modalities. CAD has long since left behind its branding as a mammography tool and has now widened its application areas, becoming a valuable tool for detection and diagnosis in cardiology.
March 19, 2012 – Individuals suffering from chronic heart failure (CHF) can look forward to the appearance of many new drug options over the next few years and place hope in a cure through future stem cell therapies, according to a new report by GlobalData.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

While risk for sudden death is increased four- to six-fold in patients who have experienced myocardial infarction, it remains challenging to identify the specific patients who will benefit from the standard-of-care therapy, namely, implantation of an implantable cardioverter defibrillator (ICD).
March 19, 2012 - Biomedical Systems, a global provider of cardiac diagnostic services and products, has introduced the TruVue Wireless Ambulatory ECG Monitoring System for the diagnosis and management of atrial fibrillation and other complex cardiac arrhythmia. TruVue's diagnostic benefits include the ability to record and wirelessly transmit every heartbeat for up to 30 days, perform advanced arrhythmia analysis and to provide immediate online access to all transmitted ECG.
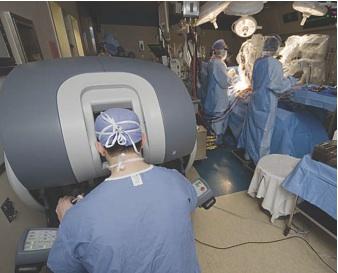
Transcatheter heart valves are viewed as the poster children devices representing the latest in cutting-edge cardiac therapy technology. For this reason, it is not surprising nearly all the readers’ nominations for DAIC’s “Most Innovative Heart Centers” included hospitals that created minimally invasive structural heart programs using the Edwards Sapien aortic valve and the Medtronic Melody pulmonary valve. Implementation of these programs usually involves the creation of a hybrid operating room (OR) and close collaboration of numerous specialists using a team approach to evaluate patients and determine the best course of treatment. Various therapy approaches are used, which can all be performed in the hybrid OR in a single surgical session.

 March 22, 2012
March 22, 2012










