The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report investigations that will be presented at next month's Transcatheter Cardiovascular Therapeutics (TCT) 2012 scientific symposium. TCT, the world's premier educational meeting specializing in interventional cardiovascular medicine, will take place Oct. 22-26 in Miami, Fla.
According to a study in the September issue of the Journal of the American College of Radiology, overall noninvasive diagnostic imaging (NDI) costs to Medicare Part B dropped 21% from 2006 to 2010. The study reveals that medical imaging is not a driver of escalating Medicare costs.
Calgary Scientific Inc. introduced ResolutionMD 3.1, a Web and mobile universal medical image viewing software that now supports 10 languages.
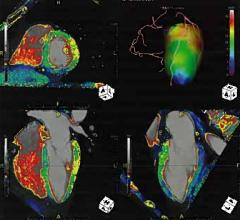
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
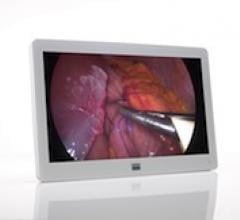
Barco announced the launch of its MDSC-2226, a 26-inch surgical display providing full compatibility with Barco’s networked digital operating room. Featuring an integrated IP-to-AV decoder, an easy-clean design and a unique cable management system, the MDSC-2226 is a perfect fit for modern digital operating rooms.

September 14, 2012 — Coronary revascularization appropriate use criteria (AUC) are now just a click away with the new SCAI Quality Improvement Toolkit (SCAI-QIT) AUC and Guidelines App, launched this week by the Society for Cardiovascular Angiography and Interventions (SCAI).
September 14, 2012 — DR Systems announced that its cloud-based ambulatory electronic health record (EHR) for radiologists, the e|HR Meaningful Use for Medical Imaging solution, is now commercially available after successfully completing beta testing.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 13, 2012 — Janssen Research & Development LLC announced that it has submitted the complete response to the U.S. Food and Drug Administration (FDA) for the use of Xarelto (rivaroxaban) to reduce the risk of secondary cardiovascular events in patients with acute coronary syndrome (ACS). The response includes specific information requested by the FDA in their letter issued to Janssen on June 21, 2012.
September 13, 2012 — SunTech Medical will display its blood pressure monitoring technology at the International Society of Hypertension (ISH) 2012 exhibition. Visitors can view the Oscar 2 ambulatory blood pressure monitoring system, as well as the SunTech 247 blood pressure and vitals spot check device at booth #18.
Austin Medical Design's Cath Cube is a single-use product for management of guidewires and catheters (balloons and stents) used in interventional cardiology and radiology labs.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 12, 2012 – Positron Corp. announced that Jubilant DraxImage Inc., a Jubilant Life Sciences Company, and Positron have executed a Letter of Intent pertaining to Positron's supply of Active Pharmaceutical Ingredient (API) grade Sr-82 for the JDI Sr-82/Rb-82 generator; JDI's and Positron's co-promotion of JDI Sr-82/Rb-82 generators to end-users (upon FDA clearance); and Positron's lifecycle management for expired JDI Sr-82/Rb-82 generators.
CircuLite Inc. announced that it has received CE marking approval for the Synergy Circulatory Support System, a micro-pump designed to halt the progression of heart failure. By working with the heart’s native pumping capacity, Synergy is specifically targeted for treatment of ambulatory heart failure patients (INTERMACS 4,5,6 / NYHA IIIB and early IV) who remain symptomatic despite standard medical management.
Abiomed Inc. announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a new percutaneous, catheter-based Impella ventricular assist device (VAD) providing peak flows of approximately 4 liters of blood per minute. The increased flow is delivered on the same console platform, 9 French catheter and introducer as the Impella 2.5. This new heart pump will be marketed as the Impella CP (Cardiac Power) within the United States and has been commercially known outside the U.S. as the Impella cVAD.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 11, 2012 — St. Jude Medical Inc. announced the realignment of its product divisions into two new operating units: the Implantable Electronic Systems Division (IESD) and the Cardiovascular and Ablation Technologies Division (CATD).
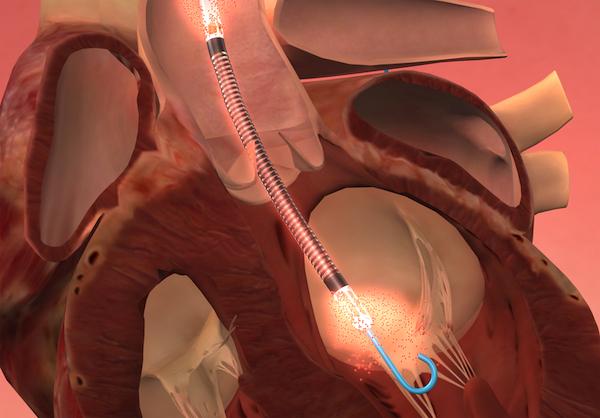
September 11, 2012 — Primary results from an ongoing trial by Maquet Cardiovascular using intra-aortic balloon pumps (IABP) as an adjuvant treatment for myocardial infarction (MI) complicated by cardiogenic shock have shown no difference in patient survival when compared to standard care alone.

September 11, 2012 — Since the previous consensus document was published in 2007, catheter and surgical ablation of atrial fibrilliation (AF) have become standard treatments and more randomized trials of ablation versus optimal drug therapy for AF have been conducted. “Significantly more data exist on techniques, success rates and complications of these new interventions, making this a more valid document compared to 2007,” said Professor Karl Heinz Kuck of Germany, president-elect of the European Heart Rhythm Association (EHRA) and co-chair of the task force that developed the latest document.


 September 17, 2012
September 17, 2012