St. Jude Medical Inc. (announced European CE mark approval of the Assura portfolio of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds). The Assura family of devices, featuring Shockguard technology has three new algorithms that help protect patients against inappropriate shocks while providing the highest amount of delivered energy. These improvements are projected to reduce inappropriate therapy by 74 percent, allowing for more effective therapy. Products currently available in Europe include the Quadra Assura CRT-D, Unify Assura CRT-D and Fortify Assura ICD.
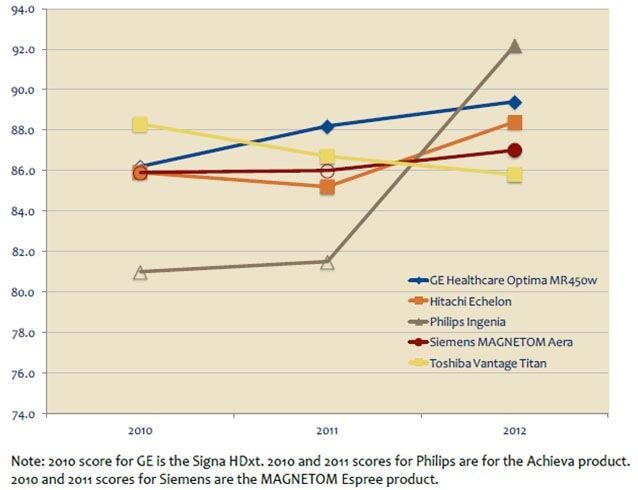
The introduction of new technology into the magnetic resonance imaging (MRI) landscape has changed the face of the market segment over the last year. Although MRI satisfaction scores tend to cluster together, the differences are in the details, according to recent findings from healthcare technology research firm KLAS in its report MRI 2012: Broadening Your Field of View.
When tests reveal that a patient’s heart artery is blocked and blood flow to the heart is restricted, should the doctor fix it with a stent or stop the test and discuss treatment options with the patient? Recommendations published by the Society for Cardiovascular Angiography and Interventions (SCAI) provide new guidance on when physicians should move forward immediately to open a blocked artery versus when to stop the test for further discussion with the patient or consultation with a heart surgeon.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
RamSoft announced its partnership with Digisonics to offer integrated, vendor-neutral cardiac post-processing and structured reporting for all cardiovascular modalities. Digisonics was recently named Best in KLAS for the Cardiology market segment for the fifth consecutive year.
According to Millennium Research Group (MRG), approximately 80 percent of transcatheter aortic valve replacement (TAVR) procedures are being performed on high-risk patients who are ineligible for surgical heart valve replacement. New clinical evidence that supports TAVR in treatment of intermediate and high-risk operable patients has led to expanded reimbursement for a new patient base for the procedure.
Merge Healthcare Inc. announced that Merge Hemo has been named the "Category Leader" in the "2012 Best in KLAS Awards: Software & Services" report for Cardiology Hemodynamics rankings for the second consecutive year.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Some stroke patients may benefit from cerebral angioplasty and stent placement, according to a new study published online in the journal Radiology.
The first patient has been implanted with the Boston Scientific Corporation next generation Ingevity pacing leads in a clinical trial designed to establish the safety, performance and effectiveness of the leads. Pacing leads are insulated wires that connect an implantable pacemaker to the heart for treatment of bradycardia, a condition in which the heart beats too slowly, depriving the body of sufficient oxygen. Pacemakers work in conjunction with leads to sense and stimulate (or pace) the heart, thus maintaining an appropriate heart rate for a given level of physical activity.
Among deployed U.S. service members who died of combat or unintentional injuries between 2001-2011 and underwent autopsies, the prevalence of coronary atherosclerosis was 8.5 percent, with factors associated with a higher prevalence of the disease including older age, lower educational level and prior diagnoses of dyslipidemia, hypertension and obesity, according to a clinical study in the Dec. 26 issue of JAMA.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Pulse Oximetry Screening is a life-saving test that can detect critical congenital heart defects (CCHD) in newborn babies before an infant is discharged from the hospital. The test is easy to perform, however, appropriate interpretation of the results can be challenging. In order to aid health care providers in interpreting the results of the screening, Children's Healthcare of Atlanta has created the Pulse Ox Tool, a ground-breaking app for smart phones that automates the Pulse Oximetry Screening test and improves the accuracy of detecting children with possible CCHD.

The past year in cardiology has continued to see progress and innovation in medicine. As the year comes to an end, the American College of Cardiology (ACC) identified some of the top cardiovascular stories of 2012.
Resuscitation, cell regeneration, a new high blood pressure treatment and developments in devices for treating stroke are among the key scientific findings that make up this year's top cardiovascular and stroke research identified by the American Heart Association and American Stroke Association.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
December 28, 2012 — Researchers have discovered that adding lovastatin, a widely used cholesterol-lowering drug, to traditional antimalarial treatment decreases neuroinflammation and protects against cognitive impairment in a mouse model of cerebral malaria.
December 27, 2012 — Cardiosonic Inc., a private developer of technology in the field of renal denervation for the treatment of hypertension, announced it has closed on the first $6.1 million of a planned $16.1 million Series B financing.
December 27, 2012 — Biotronik announced that the first implant has taken place in the BIOHELIX-I trial in the United States. The BIOHELIX–I trial is set to evaluate the safety and performance of the Pro-Kinetic Energy coronary bare metal stent.


 December 31, 2012
December 31, 2012