Peri-natal systems real-time clinical decision support company PeriGen Inc. announced the results of an independent validation study on fetal ECG strip interpretation favorably comparing PeriGen PeriCALM Patterns software with the findings of three experts from the National Institute of Health (NIH). The U.S. Food and Drug Administration (FDA)-cleared fetal strip interpretation technology is a component of the company's perinatal central surveillance offering.

Using tiny balloons to temporarily block blood flow to the uterus during a high-risk Caesarean-section delivery can save the life of the mother while preventing hysterectomy and preserving fertility, suggests research being presented at the 25th annual International Symposium on Endovascular Therapy (ISET).
Cordis Corporation announced the presentation of the two-year STROLL study results at the Abstracts and Late Breaking Clinical Trials session at ISET 2013.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Biotronik announced that the U.S. Food and Drug Administration (FDA) has approved, with conditions, the ProMRI clinical trial via an Investigational Device Exemption. Biotronik is sponsoring the trial (NCT01761162) and has already initiated U.S. site recruitment. The ProMRI clinical trial is designed to recruit 245 subjects implanted with one of Biotronik’s cardiac devices that include ProMRI technology at 30 U.S. investigational centers.
GNAX Health announced the availability of GNAX SDEX Secure digital imaging and communications in medicine (DICOM) Exchange, allowing hospitals, ACOs and HIEs the ability to transfer medical images securely. The announcement was made by Jeff Hinkle, CEO, GNAX, at the Radiological Society of North America's 98th Scientific Assembly and Annual Meeting in Chicago, Ill.
January 21, 2013 — ECRI Institute, a nonprofit organization that researches the best approaches to improving patient care, recently evaluated evidence behind the use of the only transcatheter heart valve approved for marketing in the United States and Europe in transcatheter aortic valve implantation (TAVI).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 21, 2013 — Avreo announced it has formed a partnership with VidiStar LLC, a provider of cardiology medical imaging and reporting solutions using the latest technological standards for image interpretation, clinical analysis and medical reporting.
The first patient has been treated in the Boston Scientific Corporation ZERO AF clinical trial to evaluate the safety and effectiveness of the Blazer open-irrigated temperature ablation Catheter in patients with symptomatic, drug refractory paroxysmal atrial fibrillation. This international, multi-center study will include up to 33 sites in the United States, Europe and Asia-Pacific, and as many as 472 patients. The results of the ZERO AF trial are expected to be used to support a U.S. Food and Drug Administration (FDA) regulatory submission for a paroxysmal atrial fibrillation indication.
At the Boston Atrial Fibrillation (AF) Symposium 2013, Philips Healthcare introduced its latest innovations in advanced imaging integration for electrophysiologists, showcasing its new EP navigator offering — a novel approach to 3-D rotational angiography that provides real-time anatomical details of cardiac LA-PV structures with significantly enhanced workflow.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
GE Healthcare announced that it has filed a supplemental new drug application (sNDA) that will allow the company to manufacture Optison (Perflutren Protein-Type A Microspheres Injectable Suspension, USP), within its own facility. Optison is a contrast agent that may improve the visualization of the left ventricular border — an area of the heart that is critical to see in order to assess and diagnose certain heart diseases. Upon approval, GE Healthcare will provide supply of Optison to the U.S. market from its manufacturing facility in Oslo, becoming the only contrast media manufacturer to supply its own stock for the United States.
nContact Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the Company’s modifications to its VisiTrax cardiac ablation device, EPi-Sense, now with embedded sensing capability. This next generation of technology includes sensors along the ablation device, which provide real-time feedback to physicians conducting cardiac ablation procedures.
CardioMEMS Inc. has been honored as the 2012 recipient of the Intel Innovation Award. Founder and CEO Jay Yadav, M.D., was presented with the award on Dec. 4, 2012 during the annual Health IT Leadership Summit held at the historic Fox Theatre in Atlanta, Ga.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
CardioKinetix Inc. announced the enrollment of the first patients in PARACHUTE IV, the randomized pivotal U.S. trial of the minimally invasive Parachute Ventricular Partitioning Device for the treatment of heart failure.
GSI Group Inc., a leading supplier of laser-based solutions, precision motion and optical technologies to global industrial, medical, electronics and scientific markets, announced that it has acquired NDS Surgical Imaging (NDS), a San Jose, Calif.-based global leader in surgical and radiology displays and related peripherals, for $82.5 million in cash, subject to customary closing adjustments.
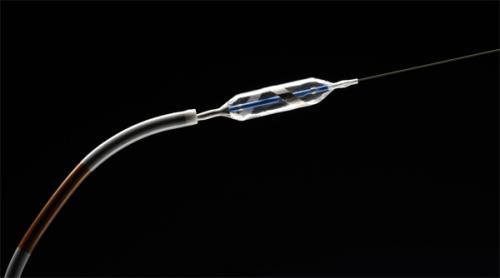
Covidien began the commercial launch of the OneShot Renal Denervation System, an over the wire balloon-based irrigated ablation catheter technology for the treatment of hypertension.

 January 22, 2013
January 22, 2013