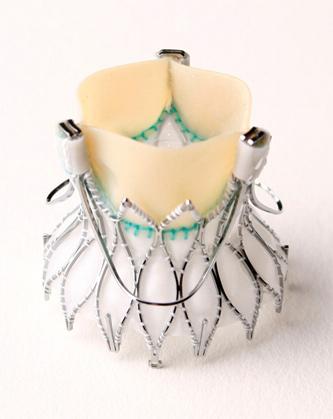
Medtronic Inc. announced European CE (Conformité Européenne) mark approval for its Engager Transcatheter Aortic Valve Implantation (TAVI) System with transapical delivery catheter. The system is indicated to treat patients with severe aortic stenosis who are at high or extreme risk for surgical aortic valve replacement (SAVR).

The Healthcare Supply Chain Association (HSCA) today launched Medical Device Tax Watch, a website dedicated to raising awareness of efforts of some medical device manufacturers to pass the costs of the medical device excise tax (MDET) to American hospitals, healthcare providers and patients. The new site will be a resource for hospitals and a tool for monitoring the device marketplace, and can be found at www.devicetaxwatch.com.
Corindus Vascular Robotics will showcase its U.S. Food and Drug Administration (FDA)-cleared CorPath 200 Vascular Robotic-Assisted System at the American College of Cardiology 2013 meeting, March 9-11.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Strain imaging for echo is increasingly being recommended to assist in assessing cancer patients at risk for heart disease. Strain Imaging can provide valuable information when monitoring patients for cardiotoxicity resulting from therapies used to treat cancer.
Schiller's new medilog AR12 plus ECG Holter recorder includes enhanced features such as P-wave detection for atrial analysis, patient movement pattern recognition by on-board three-axis accelerometers, and special purpose noise suppression circuitry optimized for electrocardiogram (ECG). The system also offers a color display,? hook-up impedance test and built-in microphone.?
The Schiller 12-Lead ECG MS-2015 unites precision, performance and a sophisticated but simplified user interface.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Mortara Instrument Inc. began its worldwide release of its latest resting electrocardiograph (ECG), the ELI 280. The new system combines advanced ECG processing with an intuitive interface, enabling clinicians optimized efficiency in their acquisition of routine diagnostic ECGs.
With Schiller's medilog electrocardiogram (ECG) Holter recorders and the new Fire of Life software, the heart rate variability (HRV) is analyzed and displayed in a new way to help judge the function of the autonomic nervous system.
Cardea Associates announced the launch of its new electrocardiographic (ECG) device, CardeaScreen. Specifically in-tune with normal conditions for the athletic heart, CardeaScreen is designed for use during pre-participation exams (PPE) for sports participants age 14 and older. This U.S. Food and Drug Administration (FDA)-cleared device helps physicians identify abnormal cardiac conditions that could lead to sudden cardiac arrest — at a much lower cost than other ECG systems currently on the market.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Laboratory Corporation of America Holdings (LabCorp) announced it has launched its clinical next generation sequencing assay, GeneSeq: Cardio for genetic causes of familial cardiac disease.
The Society of Cardiovascular Computed Tomography (SCCT) has released a list of five interventions whose appropriateness physicians and patients should discuss as part of Choosing Wisely. The list includes:

The Society of Nuclear Medicine and Molecular Imaging (SNMMI) released a list of specific tests that are commonly ordered—but not always necessary — in nuclear medicine and molecular imaging as part of the Choosing Wisely campaign, an initiative of the ABIM Foundation. The list identifies five targeted, evidence-based recommendations that can support conversations between patients and physicians about what care is really necessary and appropriate.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 26, 2013 — TeraMedica debuted the Evercore Connext Mobile at the 2013 HIMSS conference, bringing groundbreaking, seamless point-of-care data capture and archiving to an iPad or iPhone. At the touch of a button, Evercore Connext Mobile acquires photos, videos, sound files, notes and more with the highest level of security. Data is automatically archived directly to the hospital’s Evercore VNA as an integral part of the patient record for viewing through an electronic medical record (EMR) and the integrated TeraMedica Univision viewer.
The TIMS Medical division of Foresight Imaging released the TIMS version 3.0 platform to provide high resolution digital video formats in fluoroscopy and endoscopy.
Medtronic Inc. announced it received U.S. Food and Drug Administration (FDA) approval to conduct an early feasibility study using the Medtronic Native Outflow Tract Transcatheter Pulmonary Valve (TPV). This approval represents the first-ever FDA approval of an investigational device exemption (IDE) following the new draft FDA guidance for early feasibility studies.

 February 28, 2013
February 28, 2013














