According to a new study published online in the Journal of the American College of Radiology, any efficiencies in physician interpretation and diagnosis gained when different providers interpret different medical imaging scans performed on the same patient are minute and vary by procedure.
MIM Software Inc. has announced its software release MIM 6 is now available. Users in all areas of medical imaging can benefit from tools for deformable evaluation and correction, magnetic resonance/computed tomography (MR/CT) deformable and automated series management.
American Well has announced a new telehealth pilot program at Massachusetts General Hospital to better monitor heart failure patients by making physicians available to patients through live, real-time video visits.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
One thousand days, 1,000 C-arms: this is the result three years after the introduction of the Ziehm Solo. As one of the most compact and flexible C-arms, the Ziehm Solo adjusts to the special demands and requirements of clinics. More and more users from orthopedics and trauma opt for Ziehm Imaging’s multi-talent for intraoperative imaging. The 1,000th Ziehm Solo will be delivered to the Clinica Santa Elena in Malaga, Spain.
Cancer survivors who had chest radiation are nearly twice as likely to die in the years after having major heart surgery as similar patients who didn't have radiation, according to research in the American Heart Association journal Circulation.
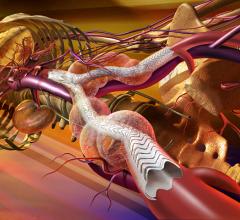
W. L. Gore & Associates Inc. has received U.S. Food and Drug Administraion (FDA) approval for the new large diameter 35 mm trunk-ipsilateral leg and 36 mm aortic extender components, as well as the lower profile 31 mm diameter trunk-ipsilateral leg and 32 mm aortic extender components of the Gore Excluder AAA Endoprosthesis. The new components provide physicians with a proven and durable endovascular option to treat abdominal aortic aneurysms (AAAs).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Tyrx Inc. has received a license from Health Canada to market its new AigisRx R Fully Resorbable Antibacterial Envelope technology with implantable electronic devices (IEDs). The AigisRx R Antibacterial Envelope is specifically designed to stabilize IEDs while also releasing antimicrobial agents to help provide protection from microbial colonization of the device during surgical implantation. Tyrx is the leader in the commercialization of implantable medical devices intended to help reduce surgical site infections.
The FREEDOM trial, the first long-term, comparative study of its kind exclusively for patients with diabetes and advanced multivessel coronary artery disease (CAD) revealed that diabetics with CAD live longer and are less likely to suffer a non-fatal heart attack when treated with bypass surgery instead of drug-eluting stents (DES), tiny, medicine-coated mesh tubes that prop open clogged arteries.
Samsung Electronics America Inc. announced the Samsung Ugeo H60, the first ultrasound system integrating Samsung technology and design introduced to the U.S. medical imaging market. The Samsung Ugeo H60 ensures superior imaging performance and a sleek design that provides users with a fast, accurate and intuitive ultrasound experience.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Angiotech Pharmaceuticals Inc. announced that it entered into a definitive agreement to sell certain of its subsidiaries, comprising Angiotech's interventional products business, to Argon Medical Devices Inc., a portfolio company of RoundTable Healthcare Partners, for $362.5 million in cash consideration. Angiotech expects the transaction will close prior to the end of April 2013.
Archimedes IndiGo — the physician and patient decision-support tool created by Archimedes, Inc. — was chosen last summer as the risk-assessment engine for the new $100,000 mobile app challenge sponsored by the Office of the National Coordinator (ONC). As part of the U.S. Department of Health and Human Services' Million Hearts "Risk Check Challenge," the ONC invited developers to create a mobile app that would help consumers take a heart-health risk assessment, direct them to health-screening locations in their community and use the results to work with their doctor to develop a plan for improving their heart health.

The American Society of Echocardiography (ASE) has released a list of five interventions whose appropriateness physicians and patients should discuss as part of Choosing Wisely, an initiative of the ABIM Foundation, along with Consumer Reports. Fifth on the list, they ask that patients and their doctors talk about the real need for a TEE if the patient’s clot treatment isn’t likely to be modified.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Terumo Cardiovascular Systems announced it has entered into a multi-year distribution agreement with Nonin Medical, Inc. Beginning May 1, 2013, Terumo CVS will distribute Nonin's Equanox Model 7600 Regional Oximetry System to adult and pediatric cardiovascular hospitals in the U.S. Nonin's direct sales force will focus on certain non-cardiovascular applications in those hospitals and applications in all other U.S. hospitals. Terumo CVS manufactures and markets medical devices for the global cardiac surgery market.
Crouse Hospital boasts a nationally recognized cardiac care center, providing a full range of interventional and diagnostic procedures for pediatric and adult patients in the greater Syracuse, N.Y., area. Its robust program requires state-of-the-art technology. Crouse recently implemented HealthView, a Web-based cardiovascular information system (CVIS) by Lumedx that streamlines clinical workflows and improves access to patient information across the care continuum.
Atomic Energy of Canada Limited (AECL) will commence a 30-day planned shutdown for inspection and maintenance of the National Research Universal (NRU) reactor at Chalk River, Ontario, starting April 14, 2013. This planned shutdown, which AECL has advised will last until May 14, 2013, will result in an interruption in the supply of medical isotopes used in nuclear imaging procedures in the United States. Chalk River is one of the primary sources for the isotopes used in nuclear imaging radiopharmaceuticals.

 April 10, 2013
April 10, 2013