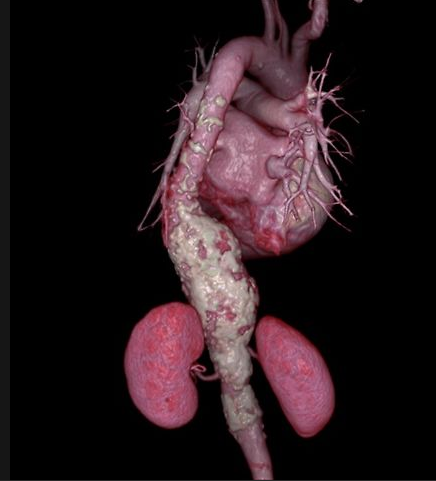
Patients with ruptured abdominal aortic aneurysms (AAA) are more than twice as likely to survive if they have minimally invasive repair than if they have surgery, suggests a 10-year study being.
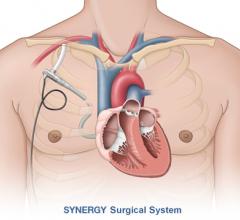
CircuLite Inc. announced that it has received approval from the Federal Agency for Medicines and Health Products in Belgium to commence the CE mark trial of the Synergy IC Circulatory Support System, the first mechanical support system that does not require major surgery. The Synergy IC System is based on the surgical Synergy Circulatory Support System — the world’s smallest commercially available circulatory support pump — which is designed to treat ambulatory chronic heart failure patients (INTERMACS ?4).
A device commonly used to treat dangerous heart rhythms may cause more issues for patients than a simpler version of the same device. The implantable cardioverter-defibrillator (ICD) prevents sudden cardiac death by detecting irregularities and delivering an electrical jolt to restart the heart.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Can high blood pressure be safely reduced and controlled by “disconnecting” nerves in the kidneys? That is a question that a new clinical study called Symplicity HTN-3 at University Hospitals (UH) Case Medical Center hopes to answer.
Patients with coronary artery disease who undergo treatment at the University of Maryland Medical Center (UMMC) now can receive long-term therapy based on information found in their genes. As part of a new personalized medicine initiative, the medical center is offering genetic testing to help doctors determine which medication a patient should take after a stenting procedure in order to prevent blood clots that could lead to serious or fatal heart attacks and strokes.

Prof. Dr. Béla Merkely and Dr. Péter Sótonyi at Semmelweis Egyetem Kardiológiai Központ in Hungary completed the first patient implant of the Barostim neo device for hypertension. Barostim neo is a small, easy to implant device manufactured by CVRx.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The Journal of American College of Cardiology has published the results from Corindus Vascular Robotics’ CorPath PRECISE (Percutaneous Robotic-Enhanced Coronary Intervention) study in the April 2013 issue. Results of the trial demonstrate the CorPath System is safe and feasible for patients, with significantly lower harmful radiation exposure to the operator.
The U.S. Food and Drug Administration (FDA) granted 510(k) clearance to Medical Imaging Electronics (MiE) and Nuclear Imaging Services (NIS) ECAT Scintron. It is the only upgrade path available to the Siemens ECAT 47, ECAT HR+, and ECAT Accel positron emission tomography (PET) systems. The ACSII and SUN workstation are removed from the system and replaced with new PC-based technology that provides new and existing users of the ECAT series PET systems access to faster processing speeds, new acquisition protocols and parts availability. With improved reliability and longevity, the ECAT Scintron redefines the life cycle of dedicated PET systems in the U.S. market.
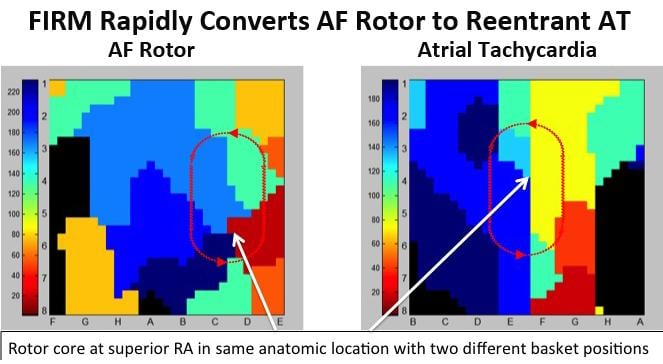
New research presented at Heart Rhythm 2013 continues to show promising results for focal impulse and rotor modulation (FIRM) mapping to effectively target atrial fibrillation (AF) sources and improves ablation therapy outcomes. The novel diagnostic real-time mapping system helps target ablation therapy to patient-specific drivers of AF rather than to anatomical targets, which can improve patient outcomes.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
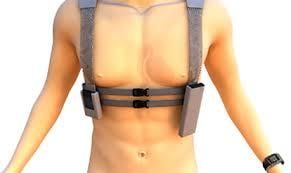
Leviticus Cardio performed a successful animal surgical trial using its wireless coplanar energy transfer system (CET) for ventricular assist devices (VAD). The surgery was performed at Assaf Harofeh Hospital and produced a 75 percent full system efficiency rate as compared with 78 percent efficiency in vitro.
The incremental mortality in implantable pacemaker and defibrillator recipients who experience a device infection, compared to patients without device infection, is substantial and persists for at least three years after index hospitalization with infection. These are the key findings of a retrospective cohort study of 200,219 Medicare fee-for-service patients undergoing cardiac device procedures, with and without infection, that were presented today by M. Rizwan Sohail, M.D., a researcher from the Mayo Clinic divisions of infectious diseases and cardiovascular diseases, at Heart Rhythm 2013, the Heart Rhythm Society’s 34th Annual Scientific Sessions.
New data from 100,438 patients with Boston Scientific implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) followed in the Latitude Patient Management System demonstrate the battery life of Boston Scientific single-chamber ICDs, dual-chamber ICDs and CRT-Ds are projected to last an average of 13.2, 11.5 and 9.2 years, respectively
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

St. Jude Medical gained CE mark approval of its Ilumien Optis percutaneous coronary intervention (PCI) optimization system to better visualize stent planning and navigation. The system integrates both fractional flow reserve (FFR) technology to measure pressure inside the coronary arteries and intravascular optical coherence tomography (OCT) imaging technology, in one system.
Positron Corp. announced the release of the PosiRx 3000-Series, its latest pharmacy automation systems. The PosiRx 3000-Series are the first systems to automate and encompass the complete compounding process, from generator elution to dose distribution, of multiple diagnostic SPECT agents in an environment engineered to be ISO Class 5 and USP 797 compliant. Designed for facilities dispensing as many as 300 patient-specific doses per day, the PosiRx 3000-Series will benefit providers and patients by enabling unit dose radiopharmaceuticals to be prepared more cost effectively and accurately than previously possible.

Elixir Medical Corp. announced it received CE (Conformité Européenne) mark approval for its DESolve Novolimus-eluting bioresorbable coronary stent scaffold system. The scaffold is designed to degrade in about one year returning the patients’ coronary vessel ultimately to its normal de novo state.

 May 20, 2013
May 20, 2013