July 25, 2013 — The 2013 ESC (European Society of Cardiology) Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy, developed in collaboration with the European Heart Rhythm Association (EHRA), have created a new classification system for bradyarrhythmias according to mechanisms rather than aetiology.

Sanford Aberdeen Medical Center in Aberdeen, S.D. became the first hospital to perform a robotic angioplasty for a patient with an acute heart attack, achieving a far better door-to-balloon time than the national standard. Interventional cardiologist Puneet Sharma, performed the percutaneous coronary intervention (PCI) to treat a patient that had experienced a heart attack and presented to the Sanford Aberdeen emergency department. Utilizing the U.S. Food and Drug Administration (FDA)-cleared CorPath System, Sharma was able to perform the robotic-assisted angioplasty procedure and restore blood flow to the patient’s heart within 68 minutes of their arrival.
July 24, 2013 — St. Jude Medical Inc. announced regulatory approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) and launch of the Accent MRI pacemaker and the Tendril MRI lead.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Infraredx Inc. announced the publication of key data supporting the ability of its TVC imaging system to detect lipid core plaque in patients with ST-segment elevation myocardial infarction (STEMI). The study, published in JACC: “Cardiovascular Interventions,” details the identification of a specific cholesterol signature by near-infrared spectroscopy (NIRS) at the site of the culprit lesions causing STEMI, a dangerous type of heart attack. While the TVC Imaging System has been used in more than 3,000 patients worldwide, the present clinical study is the first report of its use in a consecutive series of STEMI patients.
July 23, 2013 — BioVentrix, known for the Less Invasive Ventricular Enhancement (LIVE) procedure for the treatment of heart failure, announced publication of baseline and 12-month comparative data demonstrating the durability of its Revivent myocardial anchoring system in the first 11 patients treated with the device.
IMRIS has announced U.S. Food and Drug Administration (FDA) 510K clearance to market VISIUS iCT, the first and only ceiling-mounted intraoperative computed tomography (iCT) on the market.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Zoll Medical Corp has announced that survival from one of the leading causes of death in the United States, out-of-hospital cardiac arrest, more than doubled from 26 percent to 56 percent when paramedics in Mesa, Ariz., utilized Zoll’s cardiopulmonary resuscitation (CPR) feedback technology in combination with scenario-based training. The findings also showed that high-quality CPR was associated with significantly improved neurologically intact survival.

Cleaning your mouth and cleaning your arteries could be as simple as a once-a-day oral rinse if additional clinical studies confirm preliminary findings about a new product. The Biomedical Development Corp. (BDC) presented data in April to the American Academy of Oral Medicine showing that its oral rinse was safe and effective at fighting gingivitis in a recent clinical trial. But the most surprising finding of the study was that users of the oral rinse showed significantly lower LDL cholesterol levels than the placebo group.

Radiation exposure from multidetector computed tomography (CT) has become a pressing public health concern in both lay and medical publications. Implementation of iterative reconstruction offers the ability to minimize radiation exposure while preserving and, in some cases, improving image quality. However, in order to evaluate iterative reconstruction software, one must first understand the basics of how it works.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
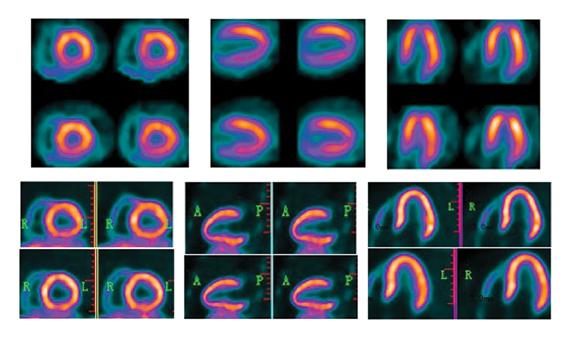
Just when positron emission tomography (PET) appears to be eclipsing single photon emission computed tomography (SPECT) for cardiac imaging, new advances make SPECT more attractive. Both modalities also have suffered setbacks with radiopharmaceutical supply problems in recent years and both modalities have their pros and cons. Looking toward the future, the question of which modality will dominate remains unanswered. PET shows major promise with exciting new tracers, while new SPECT scanner technology introduced at the Society of of Nuclear Medicine and Molecular Imaging (SNMMI) 2013 meeting may herald a rebirth for SPECT with previously unseen image quality enhancements.

Percutaneous transluminal coronary angioplasty (PTCA) balloon catheters were the first devices used in the field of interventional cardiology. The growth of the stent market, which is the largest segment for interventional cardiology devices, has helped to fuel the sales of PTCA balloons. In addition, this market is driven by U.S. Food and Drug Administration (FDA) policy, pre-/post-stent dilations and plaque debulking.

Computerized and semi-automated inventory control system technology offers a more efficient way for cardiovascular departments, especially catheterization and electrophysiology (EP) labs, to track what is on their shelves, when to re-order supplies, their actual costs per procedure and to identify new ways to cut costs.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Healthcare reform requiring wider access and enterprise sharing of patient images and records are making Web-based cardiology picture archiving and communication systems (PACS) a more attractive solution over traditional thick-client, server-based systems. In just the past few years there has been a departure from thick-client cardiology and radiology PACS to Web-based platforms. There are several reasons for this, including better interoperability, anywhere-anytime access, remote access to data and images, and reduction of IT burdens. Web-based systems also enable easier delivery of many healthcare reform Stage 2 meaningful use (MU) requirements.
Finding ways to lower patient radiation dose from both medical imaging and interventional cardiology has become a major trend. However, when vendors start talking dose, it is important to realize there are no set industry standards agreed upon by manufacturers to calculate dose. For this reason, I call into question vendors’ statistics of how much their technology can lower dose by up to 20, 50 or even 80 percent. While new technologies such as iterative reconstruction, more sensitive detectors and ECG gating do indeed lower dose, quantifying it can be a moving target.

AngioScore Inc. announced the completion of enrollment in the Drug-Coated AngioSculpt Scoring Balloon Catheter First-in-Human (FIH) Study (PATENT-C). AngioScore anticipates that preliminary data from this study will be available for presentation at the Transcatheter Cardiovascular Therapeutics (TCT) conference to be held this fall in San Francisco


 July 25, 2013
July 25, 2013







