Among patients with prehypertension and coronary artery disease, use of the renin (an enzyme secreted by the kidneys) inhibitor aliskiren, compared with placebo, did not result in improvement or slowing in the progression of coronary atherosclerosis, according to a study published by JAMA. The study was released early online to coincide with its presentation at the European Society of Cardiology Congress 2013.

Measuring the benefits against the risks (and costs) is an integral component of a sound business plan and will assist in the design, development and deployment of a successful cardiovascular information system (CVIS) strategy.
Reportlinker.com released a new report on the MRI market titled “Advances in Magnetic Resonance Imaging (MRI) Market (2013-2018) - Technology Trend Analysis – By Architecture, Field Strengths, Technology & Applications in Medical Diagnostics with Market Landscape Analysis – Estimates up to 2018.”
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Physio-Control launched its comprehensive solution designed to improve cardiac resuscitation in hospitals. The CodeManagement Module, which adds capnography and wireless data capabilities to the Lifepak 20/20e defibrillator/monitor platform, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) last week and achieved the CE mark for commercialization worldwide in April.
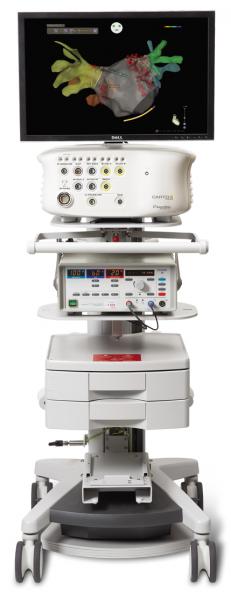
Nationwide data show that although only about 12 percent of X-ray exams are for interventional cardiology or electrophysiology (EP) procedures, nearly 50 percent of a patient’s lifetime radiation exposure comes from the cardiovascular labs. In an effort to reduce this level of patient radiation exposure, Scripps Prebys Cardiovascular Institute, part of Scripps Memorial Hospital and Scripps Green Hospital, evaluated Biosense Webster’s CartoUnivu module for the Carto 3 System (Figure 1) before it was made commercially available earlier this year. They looked for its potential benefits in reducing radiation exposure to patients and clinical staff.
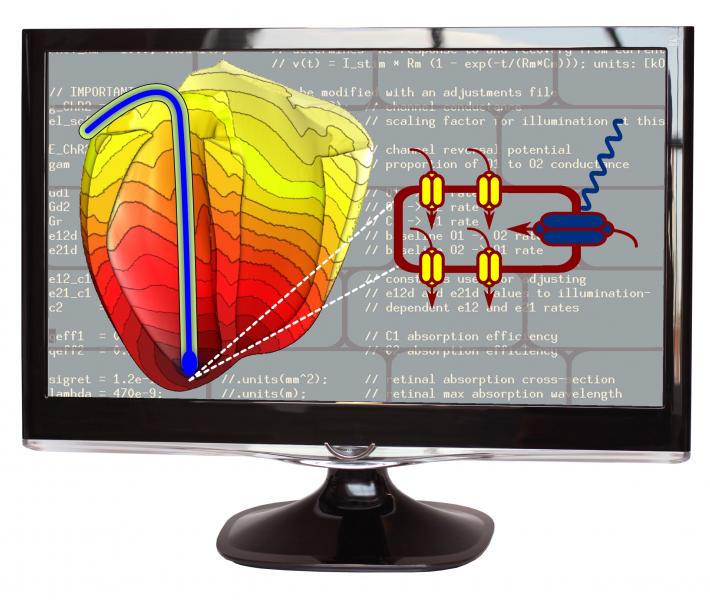
When a beating heart slips into an irregular, life-threatening rhythm, the treatment is well known: deliver a burst of electric current from a pacemaker or defibrillator. But because the electricity itself can cause pain, tissue damage and other serious side-effects, a Johns Hopkins-led research team wants to replace these jolts with a kinder, gentler remedy: light.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Philips showcased several new products at the European Society of Cardiology (ESC) 2013, including a new stress test system and a new cardiac PACS solution.
Use of the novel anticoagulant otamixaban did not reduce ischemic events compared with unfractionated heparin plus eptifibatide but increased bleeding among patients with non–ST-segment elevation acute coronary syndromes undergoing a percutaneous coronary intervention (PCI; procedures such as balloon angioplasty or stent placement used to open narrowed coronary arteries), according to a clinical study published by JAMA. The study was released early online to coincide with its presentation at the European Society of Cardiology Congress 2013.
Transcatheter aortic valve implantation (TAVI) is feasible in patients with bicuspid aortic valve (BV), according to research presented at European Society of Cardiology (ESC) Congress 2013 by Dr. Timm Bauer from Germany. The findings open up a new treatment possibility in patients with BV, which has been considered a contraindication for TAVI.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 5, 2013 — Scientists at Rice University have trapped bismuth in a nanotube cage to tag stem cells for X-ray tracking. Details of the work by a team from Rice and collaborators at the University of Houston, St. Luke's Episcopal Hospital and the Texas Heart Institute appear in the Journal of Materials Chemistry B.
Researchers announced this week that an attempt to expand cardiac resynchronization therapy (CRT) to include more patients with heart failure has failed and an international clinical trial ended early to prevent potential harm to patients.
September 5, 2013 — ITC Nexus Holding Co., a company in hemostasis management and point-of-care (POC) testing, announced it has acquired Accumetrics, a specialist in assessing patient response to all major antiplatelet therapies. Terms of the transaction were not disclosed.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
HeartWare International Inc. announced that the U.S. Food and Drug Administration (FDA) has approved an IDE (Investigational Device Exemption) Supplement that allows HeartWare to commence enrollment in an additional patient cohort for ENDURANCE, the company's pivotal, destination therapy clinical study.
Terumo has introduced the GlideSheath Slender Hydrophilic Introducer Sheath, a 6 French sheath that has the outer diameter of a 5 French sheath. Its narrower profile makes it optimal for radial access, especially in women with smaller radial access.
C. R. Bard Inc. has entered into a definitive merger agreement to acquire Rochester Medical Corp. at a price of $20 per share, or approximately $262 million in the aggregate. The Rochester Medical board of directors unanimously approved the agreement and will recommend that the company's shareholders approve the transaction.

 September 09, 2013
September 09, 2013










