
In an effort to guide regulation of mobile medical apps, the U.S. Food and Drug Administration (FDA) issued a final guidance document for developers of mobile medical apps.

Medicare expenses for patients with acute myocardial infarction (AMI, heart attack) increased substantially between 1998 and 2008, with much of the increase coming in expenses 31 days or more after the patient was hospitalized, according to a study published by JAMA Internal Medicine, a JAMA Network publication.

Lead study investigators from Icahn School of Medicine at Mount Sinai presented their Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients (PARIS) study findings at the ESC Congress 2013 in Amsterdam organized by the European Society of Cardiology. Their new study results show among patients undergoing percutaneous coronary intervention (PCI) with stents, the risk of cardiovascular complications after stopping dual antiplatelet therapy (DAPT) is highly variable depending on the context, and some patients experience no complications at all.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Siemens’ PETNET Solutions has announced a three-year agreement with The US Oncology Network to supply its entire portfolio of Food and Drug Administration (FDA)-approved positron emission tomography (PET) radiopharmaceutical agents at each of PETNET Solutions’ current Good Manufacturing Practices (cGMP)-certified locations throughout the United States.
Biotronik announced the highly-anticipated results of the Reform study. The study demonstrates that ICD (implantable cardioverter-defibrillator) patients with Biotronik's Home Monitoring can enjoy a reduction in follow-up visits by 58 percent. This in turn leads to patients reporting enhanced quality of life.
In 1988, the first Transcatheter Cardiovascular Therapeutics conference was held with less than 200 attendees in the basement of the Omni Shoreham Hotel in Washington, DC. The first meeting, a forum where physicians came to exchange ideas and compare emerging technologies, featured a single live case transmitted from Georgetown University School of Medicine.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The U.S. Food and Drug Administration (FDA) announced a final rule for the unique device identification system (UDI) that, once implemented, will provide a consistent way to identify medical devices.
A new study of patients who died of sudden cardiac arrest, a usually fatal condition that causes the heart to stop beating, shows the majority who qualified to receive potentially lifesaving treatment did not receive it.
Physio-Control launched its comprehensive solution designed to improve cardiac resuscitation in hospitals. The CodeManagement Module, which adds capnography and wireless data capabilities to the Lifepak 20/20e defibrillator/monitor platform, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in August and achieved the CE mark for commercialization worldwide in April.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Researchers have determined that fingerstick cardiac troponin I assay testing using the point-of-care i-STAT device is not accurate enough to determine the exact troponin level without the application of a corrective term.
W. L. Gore & Associates has enrolled the first patient in its Gore Carotid Stent clinical study for the treatment of carotid Artery stenosis in patients at increased risk For adverse events From CarOtid enDarterectomy (SCAFFOLD). The patient was successfully treated by Claudio Schönholz, M.D., and his team at the Medical University of South Carolina in Charleston.
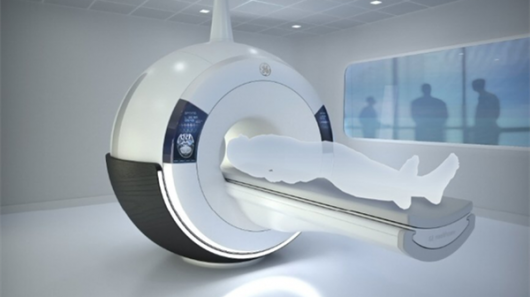
GE Healthcare reported that Silent Scan, a revolutionary technology that dramatically quiets magnetic resonance imaging (MRI) exams, is now commercially available and growing in clinical adoption around the world. Silent Scan addresses one of the most significant impediments to patient comfort — excessive acoustic noise generated during an MRI scan. Conventional MRI scanners can generate noise in excess of 110 decibels, roughly equivalent to rock concerts and requiring ear protection. GE’s exclusive Silent Scan technology is designed to reduce MR scanner noise to ambient (background) sound levels and improve a patient’s MRI exam experience.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
ResMed announced at the ESC Congress 2013 that SERVE-HF has completed enrollment.1 SERVE-HF is an international, randomised study of 1,325 participants investigating if the treatment of central sleep-disordered breathing (central sleep apnea) improves survival and outcomes of patients with stable heart failure.
Edwards announced a new post-hoc data analysis from The PARTNER Trial demonstrated that diabetic patients with aortic stenosis in need of heart valve replacement, but at high surgical risk, experienced a 35 percent lower relative all-cause mortality one year after treatment with transcatheter aortic valve replacement (TAVR), as compared to those treated with surgical aortic valve replacement (AVR).
In an independent study presented during the North American Spine Society (NASS) Annual Meeting in October 2012, GE Healthcare’s OEC Elite 9900 mobile C-arm was rated the best in image quality and dose management.

 September 26, 2013
September 26, 2013









