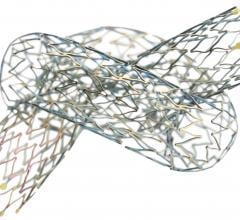
Micell Technologies Inc. announced that a peer reviewed article discussing imaging and clinical results of the Dessolve I trial of its MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES) was accepted for publication on the JACC Cardiovascular Interventions website. The paper, "First-in-Human Evaluation of a Bioabsorbable Polymer–Coated Sirolimus-Eluting Stent: Imaging and Clinical Results of the Dessolve I Trial (DES With Sirolimus and a Bioabsorbable Polymer for the Treatment of Patients With De Novo Lesion in the Native Coronary Arteries)", is planned to also appear in the October 2013 issue of JACC Cardiovascular Interventions.
Biotronik announced it has completed patient enrollment in the iliac arm of its Bioflex-I trial. Under the supervision of Mark Burket, M.D. and chief, Cardiovascular Division at the University of Toledo Medical Center, the trial will evaluate the safety and effectiveness of the Astron and Pulsar-18 stents in the treatment of peripheral vascular disease with a view towards gaining U.S. Food and Drug Administration (FDA) approval.
Imris announced U.S. Food and Drug Administration (FDA) clearance to market Visius iCT, the first and only ceiling-mounted intraoperative computed tomography (iCT) on the market.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The World Molecular Imaging Society (WMIS) held its sixth annual congress in Savannah, Ga. September 18-21. The World Molecular Imaging Congress (WMIC) 2013 featured advancements in molecular multimodal imaging with a focus on discovery, pre-clinical and translational research, highlighting first-in-human studies that drive innovative diagnostics and targeted therapy critical to the implementation of precision medicine.
At the University of Southern California Body Computing Conference, AliveCor Inc. announced its new versatile Heart Monitor, a device that easily attaches to any compatible smartphone or smartphone case.
Royal Philips and Accenture announced the creation of a proof-of-concept (POC) demonstration that uses a Google Glass head-mounted display for researching ways to improve the effectiveness and efficiency of performing surgical procedures. The demonstration connects Google Glass to Philips IntelliVue Solutions and proves the concept of seamless transfer of patient vital signs into Google Glass, potentially providing physicians with hands-free access to critical clinical information.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Emergency surgery and advanced cardiac disease are risk factors for major adverse cardiac events (MACE) after noncardiac surgery in patients with recent coronary stent implantation, according to a study published by JAMA.
Merit Medical Systems Inc. announced that it has acquired Datascope Corp.’s Safeguard Pressure Assisted Device, which assists in obtaining and maintaining hemostasis after a femoral procedure and the Air-Band Radial Compression Device, which is indicated to assist hemostasis of the radial artery puncture site while maintaining visibility.
Merit Medical Systems Inc. announced it has acquired the assets of Radial Assist, which include the Rad Board, Rad Board Xtra, Rad Trac and Rad Rest devices.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Claron Technology will announce extended modality support for its Nil universal zero-footprint medical viewers at the Radiological Society of North America (RSNA) 2013 conference.
With healthcare providers under continuing pressure to provide better care at lower costs, remote cardiac monitoring devices are becoming popular and valuable patient care tools, fueling U.S. market growth expected to top more than 25 percent between 2011 and 2016.
Image Wisely recently launched its first Image Wisely Radiation Safety Case — a series of free, online and mobile-compatible educational offerings that allow radiologists, imaging technologists and medical physicists to assess their own understanding of important radiation safety concepts such as radiation dose monitoring and optimization. The first case, CT Dose and Size-Specific Dose Estimate (SSDE), offers a total of 0.5 AMA PRA Category 1 Credits, 0.5 MPCEC credit by the Commission on Accreditation of Medical Physics Education Programs Inc. and 0.5 Category A credit hours of the American Registry of Radiologic Technologists.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Eizo Corp. and Stryker Corp. announced their partnership to offer customers integrated large monitor management systems suitable for use in standard and hybrid operating rooms. As a result of this partnership, Eizo will provide large monitors that can display multiple video sources simultaneously in conjunction with Stryker integrated operating suites. These configurable solutions will initially be offered in North America. Eizo’s platform includes large, medical-grade, high-resolution monitors up to 60 inches and the accompanying management system designed to allow users to change custom layouts
Physicians at the Heart Hospital of Austin, enrolled and successfully treated the first patient in the Roadster study, a global, multicenter clinical trial evaluating the safety and efficacy of the Silk Road System for the treatment of carotid artery disease. Mazin I. Foteh, M.D., a vascular surgeon and principal investigator for the trial, performed the first procedure.
A telestroke service increases the rate of effective tissue plasminogen activator (tPA) therapy for patients with acute ischemic stroke treated at community hospitals, according to a report in the October issue of Neurosurgery.

 October 08, 2013
October 08, 2013