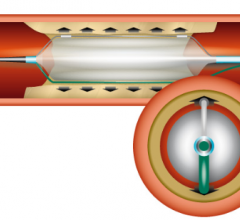
Amaranth Medical, a privately held medical device company, presented positive six-month angiographic results from its first-in-human study including patients undergoing percutaneous coronary intervention (PCI) with single coronary lesions.

The CoreValve U.S. pivotal trial showed very positive results for the Medtronic CoreValve system, comparable or better than data from the Sapien Partner Trial.
Terumo Interventional Systems announced the launch of the 0.018-inch Glidewire Advantage peripheral guidewire during the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Philips and Infraredx Inc. announced a non-exclusive resale agreement for Infraredx’s TVC imaging system. Under the terms of the agreement, Philips will sell Infraredx’s TVC imaging system alongside its Allura interventional X-ray systems in North America and Europe.
The findings of a Harvey L. Neiman Health Policy Institute study published online in the Journal of the American College of Radiology (JACR) confirm a major shift in practice at American hospitals regarding central venous procedures.
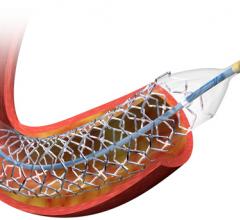
OrbusNeich announced the publication of a study demonstrating that lesion preparation with the company’s Scoreflex coronary dilatation catheter prior to drug eluting stent (DES) implantation is associated with equivalent acute stent expansion and less in-stent late loss versus a non-compliant balloon.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Nonin Medical Inc., the inventor of finger pulse oximetry and a leader in noninvasive medical monitoring, announced that the U.S. Food and Drug Administration (FDA) has cleared the Nonin Model 3230 Bluetooth Smart finger pulse oximeter for use in the United States.
Biotronik Japan announced enrollment of the first patient in the Bioflow-IV study, which aims to verify the efficacy and safety of the Orsiro Hybrid Drug-Eluting Stent (DES) from Biotronik.
The American Society of Echocardiography (ASE) Foundation is announced the Echo AUC, a new mobile application that is designed to guide physicians in appropriate procedures in cardiovascular care and echocardiography, is available for download in both the Apple and Droid application stores.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Alivecor Inc. announced that the AliveCor Heart Monitor is now being offered to participants in the Health eHeart Study with 2,000 units to be deployed before the end of the year.
Researchers at The University of Texas Health Science Center at Houston (UTHealth) led a study that showed that a hands-free ultrasound device, combined with a clot-busting drug, was safe for ischemic stroke patients.
Biosensors International’s expanded product portfolio offers physicians a broader range of treatment options to improve patient outcomes.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Simbionix, a provider of innovative training solutions, announced the initiation of the REHEARSAAAL study which evaluates the clinical benefits of preoperative patient specific simulation using the PROcedure Rehearsal Studio for the treatment of Abdominal Aortic Aneurysms (AAAs) by Endovascular Aortic Repair (EVAR). A multidisciplinary group of physicians from vascular surgery, interventional radiology and cardiology departments in hospitals across the United States and Europe will conduct the study.
CBSET, a not-for-profit preclinical research institute dedicated to biomedical research, education and advancement of medical technologies, will present data from the podium at the Transcatheter Cardiovascular Therapeutics annual scientific meeting (TCT 2013) that provides a seminal experimental and computational template for a more rational, comprehensive preclinical evaluation and optimization of renal denervation devices.
A new valve recently approved by the U.S. Food and Drug Administration (FDA) now gives heart patients a new way to manage their disease without having to undergo open heart surgery.?


 October 31, 2013
October 31, 2013