Siemens Healthcare introduced an angiography system optimized for broad clinical utilization at the Radiological Society of North America Annual Meeting (RSNA 2013).
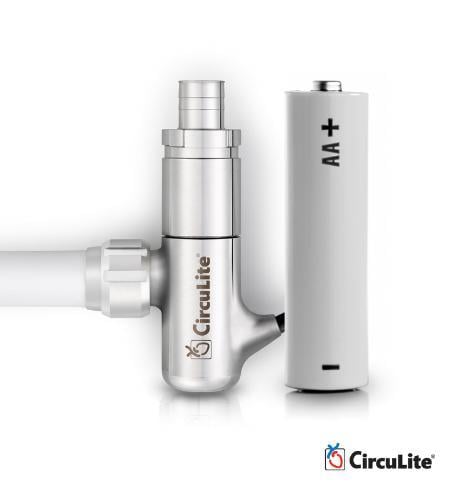
HeartWare International Inc. has acquired CircuLite, Inc., a developer of the Synergy Circulatory Support System, which is designed to treat less sick, ambulatory, chronic heart failure patients who are not yet inotrope-dependent.
Over the past decade, medical imaging has gone from being one of the fastest growing categories of Medicare spending to one of the slowest relative to other Medicare services, according to a new study.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Medtronic Inc. announced CE mark in Europe and Therapeutic Goods Administration (TGA) listing in Australia for its flexible 4 French multi-electrode Symplicity Spyral catheter and Symplicity G3 radio frequency (RF) generator.
Medtronic, Inc. presented three-year data from SYMPLICITY HTN-2, the first and longest-running randomized, controlled clinical trial of renal denervation, which continue to demonstrate results consistent with data reported previously at six, 12 and 24-months of follow-up. The data were presented for the first time during an oral abstract session on Tuesday, October 29, 2013 at the 25th Annual Transcatheter Cardiovascular Therapeutics (TCT) Symposium taking place this week in San Francisco. The Symplicity renal denervation system is available for investigational use only in the United States.
A national group of leading scientists, including one University of Alabama at Birmingham (UAB) expert, said that for more than 100 years, fewer people have been dying of stroke, yet it is still unclear why this decline remains constant.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Sidra Medical and Research Center, a specialty women's and children's hospital in Qatar, announced that Ziyad Hijazi, M.D., has been appointed clinical chief for pediatrics.
Merge Healthcare is showcasing its new universal viewer solution at Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
The Cardiothoracic Surgical Trials Network (CTSN), whose Data and Clinical Coordinating Center is at Icahn School of Medicine at Mount Sinai, is reporting evidence on whether or not there is any significant difference between the two current surgical approaches to treat patients with severe ischemic mitral regurgitation: mitral valve repair and mitral valve replacement.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Four cardiovascular professional societies released an overview of transcatheter therapies for mitral regurgitation. Intended to “help frame subsequent discussions” among the field’s various stakeholders, the document highlights critical issues that should be considered as the technologies are integrated into clinical practice.
Claron Technology, Inc. debuts version 3.0 of Withinsight Framework (WIF), an advanced platform that accelerates development of medical image visualization applications, at the 2013 Radiological Society of North America (RSNA) conference. This next-generation WIF includes enhancements in rendering, segmentation tools, and overall performance, providing Claron’s partners with advanced technology to meet their evolving needs.
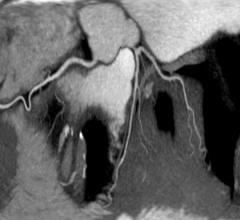
An analysis of data from an international multicenter study of coronary computed tomography angiography (CCTA) reveals that men and women with mild coronary artery disease (CAD) and similar cardiovascular risk profiles share similar prognoses.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
With the benefits of conventional strategies for therapeutic hypothermia following cardiac arrest being called in to question following the publication of two new studies, brain protection specialist BeneChill has restated its conviction that early, targeted brain cooling is the key to improved neurological outcome and survival for the estimated 700,000 people in Europe who annually suffer cardiac arrest.

Topera Inc. shared the results of an independent evaluation of its diagnostic system’s ability to improve atrial fibrillation (AF) outcomes by identifying of the sources that sustain complex cardiac arrhythmias.
Boston Scientific Corporation has received U.S. Food and Drug Administration (FDA) approval for the Promus Premier Everolimus-Eluting Platinum Chromium Coronary Stent System, the company's durable polymer drug-eluting stent (DES).

 December 06, 2013
December 06, 2013