BioVentrix’s Revivent-TC Ventricular Enhancement System was implanted in a man via Less Invasive Ventricular Enhancement (LIVE) in Prague, Czech Republic. The procedure, which is used to reshape and reduce the left ventricle, was performed on a 64-year-old man suffering from ischemic heart failure.
BeneChill Inc. received the 2014 Frost & Sullivan Award for Technology Innovation Leadership for its RhinoChill intranasal cooling system. BeneChill's RhinoChill is a nasopharyngeal device that can induce and sustain therapeutic hypothermia during active resuscitation after cardiac arrest. The system is noninvasive, time critical and can be used by nonmedical personnel to prevent brain damage associated with cardiac arrest.

As the world becomes more wired and mobile devices offer instant access to email, apps and the Web, it is only natural this technology is migrating into the healthcare sector, where physicians are seeking workflows similar to what they are familiar with when using their devices during free time. In addition to anywhere access to patient records, mobile devices can enable new functionality, such as critical results communication, mobile radiology image viewing for referring physician access, electrocardiogram (ECG) waveform access for immediate ST segment elevation myocardial infarction (STEMI) or other emergency assessment, and the ability to view completed reports on the go.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Computed Tomography (CT) procedure rates have declined by an average annual 5.5 percent in the United States over the past two years, diverging from a previous decade-long growth trend, according to a survey conducted by IMV Medical Information Division.

The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) 2013 offers many new insights about the latest cardiovascular technologies and treatment techniques. These are my choices for the most innovative new or futuristic technologies highlighted on the show floor or in sessions at TCT 2013.
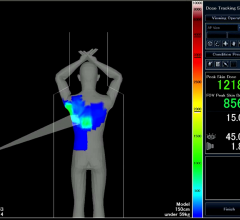
Toshiba America Medical Systems’ Dose Tracking System allows clinicians to track x-ray skin dose exposure in real time during interventional procedures.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A new internal defibrillator that treats patients with major heart risks, is being used at Henry Ford Hospital, Detroit.
Through the existing Innovator in Residence (IIR) program, HIMSS and the U.S. Department of Health and Human Services (HHS) are collaborating to move forward on the creation of a nationwide patient data matching strategy.
The U.S. Food and Drug Administration (FDA) granted U.S. market approval for Medtronic’s self-expanding transcatheter aortic valve replacement (TAVR) CoreValve System. It is the first self-expanding TAVR valve to be approved in the United States and the second TAVR valve to achieve FDA approval.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Cardiothoracic and Vascular Surgeons (CTVS) is the first practice in Texas to implant a Sorin Perceval S self-anchoring aortic heart valve as part of a new clinical trial.
Providers report that despite vendor efforts, clinical decision support (CDS) reference products are still not integrated enough to deliver the strategic direction providers are looking for.

In a move that calls into question the future of transcatheter valve competition in the United States, a jury in the Federal District Court of Delaware decided Jan. 15 the Medtronic CoreValve infringes on transcatheter device patents held by Edwards Lifesciences Corp. Medtronic said it intends to appeal the decision.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A new “fluid biopsy” technique that could identify patients at high risk of a heart attack by identifying specific cells as markers in the bloodstream has been developed by a group of researchers at The Scripps Research Institute (TSRI).
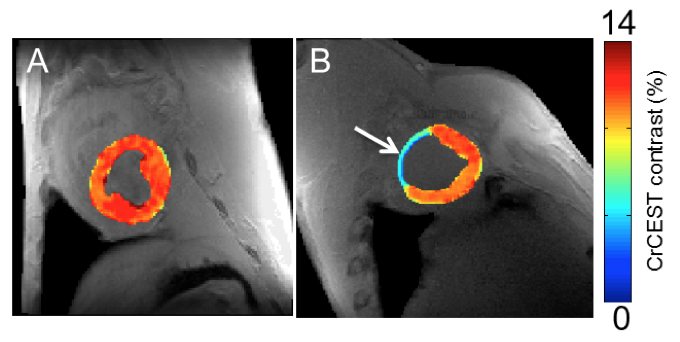
A new magnetic resonance imaging (MRI) method to map creatine at higher resolutions in the heart may help clinicians and scientists find abnormalities and disorders earlier than traditional diagnostic methods.
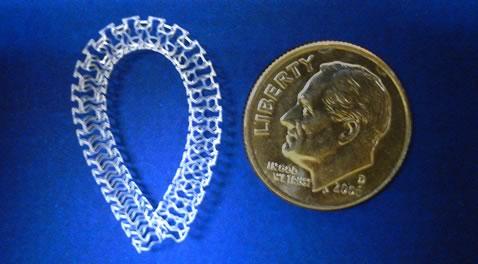
Amaranth Medical announced the closing of an equity investment by Boston Scientific Corp.

 January 22, 2014
January 22, 2014