eCardio Diagnostics is opening its second Independent Diagnostic Testing Facility (IDTF) in San Francisco, Calif. Its first IDTF is located in Houston, Texas.
Results from several late breaking clinical trials will be presented during the American College of Cardiology (ACC) 2014 annual meeting March 29-31. These will be featured during five late-breaking clinical trial sessions.
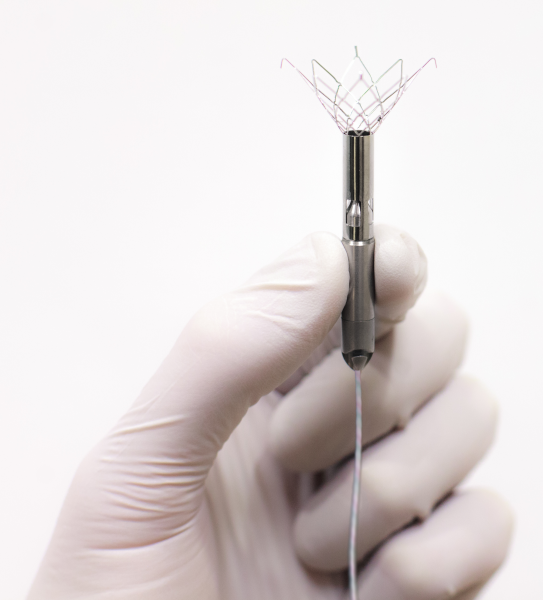
Start-up company Procyrion Inc. is developing a catheter-deployed circulatory assist device intended for long-term use in the treatment of chronic heart failure. The 6 mm diameter Aortix device is narrower than a pencil and is delivered via a catheter in a minimally invasive outpatient procedure lasting about 10 minutes.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The American College of Radiology (ACR) strongly supports the bicameral, bipartisan legislation to replace the sustainable growth rate (SGR) payment formula. The organization particularly applauds inclusion of several ACR backed provisions that raise quality of care, make care more efficient and increase transparency in physician payment policy.

Doctors at Henry Ford Hospital in Detroit used an Edwards Lifesciences Sapien transcatheter aortic valve replacement (TAVR) device to repair a mitral valve that was narrowed with calcium buildup. William O'Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, estimates this new technique could help thousands of patients a year in the United States.
Transcatheter Technologies GmbH, a medical device company, is developing a third-generation transcatheter aortic valve implantation (TAVI) system, Trinity. An independent laboratory completed advanced wear testing (AWT) of the company’s Trinity valve prosthesis. AWT of the Trinity heart valve has completed 600 million cycles, or an estimated 15 years of durability testing.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The topic of radiation safety and radiation dose monitoring has moved from state-specific regulations to a national trend with The Joint Commission’s (TJCs) recent announcement of their “New and Revised Diagnostic Imaging Standards.” The call for dose management and tracking has graduated from being advised to being mandated – from both a legal perspective and from within the world of healthcare’s patient safety foundation. The question that many organizations find themselves asking is “Where does this leave me?” and “Are we prepared for compliance?”
To address the needs of physicians who treat patients with valvular heart disease, Esaote North America established a sales force to bring 3mensio Structural Heart software for cardiovascular planning solutions.
Boston Scientific launched in the United States the OffRoad Re-Entry Catheter System to treat complete arterial blockages in the major arteries of the legs. Chronic total occlusions (CTOs), are associated with advanced peripheral artery disease (PAD).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
New electrophysiology (EP) ablation mapping/navigation systems recently entered the U.S. market, each offering technology the vendors say will speed procedure time and improve procedural accuracy.
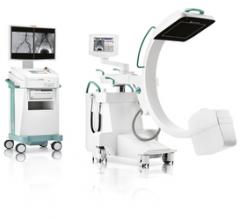
Ziehm Imaging received U.S. Food and Drug Administration (FDA) clearance to market its new generation Ziehm Vision RFD C-arm for pediatric and other interventional operating room procedures. The Ziehm Vision RFD C-arm offers fully motorized movement on four axes to maximize image quality while minimizing procedural dose. It was cleared for a range of image procedures including specific intended uses in pediatric imaging.
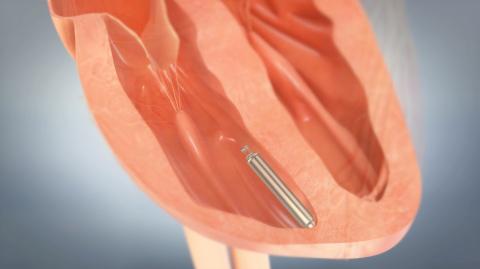
The first U.S. implant was announced for St. Jude Medical’s LEADLESS II pivotal trial, designed to evaluate the Nanostim leadless pacemaker for U.S. Food and Drug Administration (FDA) approval. The world’s first retrievable, non-surgical pacemaker was implanted at The Mount Sinai Hospital in New York City by Vivek Reddy, M.D.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The average age of installed MRI scanners in the United States has increased from 8.7 years in 2010 to 11.4 years in 2013, according to a new market research report by IMV Medical Information Division.
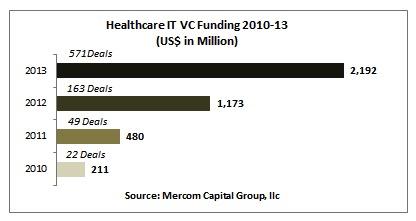
Mercom Capital Group LLC, a global communications and consulting firm, released its annual report on funding and mergers and acquisition (M&A) activity for the healthcare information technology (IT) sector in 2013.

GlobalMed’s CapSure Cloud application can eliminate a second radiation dose by making an initial CT scan available to all healthcare providers involved in a patient’s care. Once the images are uploaded to the secure cloud imaging server from the originating hospital, the sign-on information can be shared with specialists at the receiving hospital so they can view the study. In emergency cases, this means the team at the receiving hospital can be prepared for the patient’s arrival and go directly to the OR.

 February 07, 2014
February 07, 2014