Extending the "Evolution of Visual Healthcare" to mobile devices, Agfa HealthCare launched its new Web-enabled mobile image management technology that brings the power of ICIS to iPhones, iPads, Web and Android mobile digital devices.
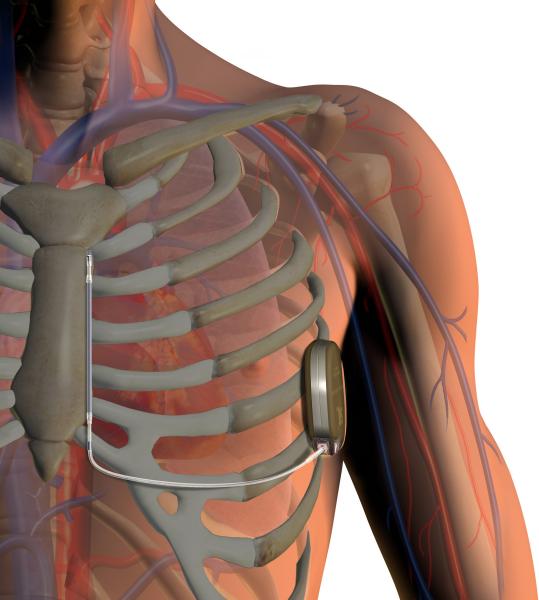
A study presented at Heart Rhythm 2014, the Heart Rhythm Society’s 35th Annual Scientific Sessions, reports significant gender and health insurance disparities in implantable cardioverter defibrillator (ICD) procedures.
Hospira Inc. issued a nationwide recall to the user level for one lot of Dobutamine Injection, USP, 250 mg, 20 mL, single-dose fliptop vial, (NDC 0409-2344-02), Lot 27-352-DK. (NDC and lot number can be found on the right-hand side of the primary label).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 21, 2014 — Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) cleared the Somatom Force computed tomography (CT) system — the next generation in dual source CT.
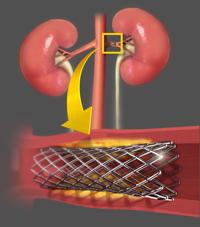
Patients with hypertension after renal artery stenting who do not respond to drug treatment may have another option.
May 21, 2014 — iRhythm Technologies Inc. announced that data from four studies presented at Heart Rhythm 2014, the Heart Rhythm Society's 35th Annual Scientific Sessions, support the use of the company's Zio Service to identify atrial fibrillation (AF) and other cardiac arrhythmias.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
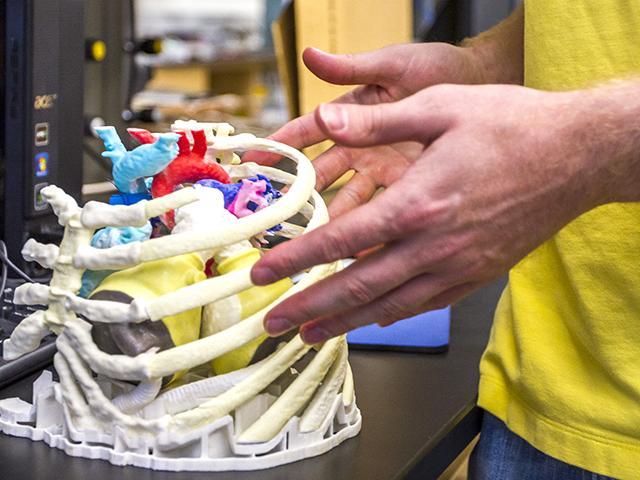
The FDA has recognized 3-D printing technology now exists to print medical devices and is gathering information regarding technical assessments that should be considered when it will inevitably begin reviewing these types of future FDA submissions.
Sony Electronics’ Medical Systems Division showcased a full line of medical grade monitors for radiology applications, including the LMD-DM50, LMD-DM30C and LMD-DM30 models, at the 2014 Society for Imaging Informatics in Medicine (SIIM) meeting, in Long Beach, Calif.

St. Jude Medical Inc. announced that the first patient implants occurred in the Portico Re-sheathable Transcatheter Aortic Valve System U.S. IDE Trial (PORTICO trial).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 20, 2014 — Siemens Healthcare announced the U.S. Food and Drug Administration (FDA) has cleared the 16- and 32-slice iterations of its Somatom Perspective computed tomography (CT) system.

May 20, 2014 — GE Healthcare and Tesla Engineering Ltd. are collaborating to produce 7T human whole-body magnetic resonance imaging (MRI) scanners. The companies made the announcement at the joint meeting of the International Society for Magnetic Resonance in Medicine (ISMRM) and the European Society for Magnetic Resonance in Medicine and Biology (ESMRMB) in Milan.
Transcatheter Technologies GmbH, an emerging medical device company that is developing a third-generation transcatheter aortic valve implantation (TAVI) system — Trinity) — announced that its founder and CEO, Wolfgang Goetz, M.D., Ph.D., will be meeting with potential investors, including potential corporate partners, at the EuroPCR annual scientific meeting in Paris, May 20-23.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
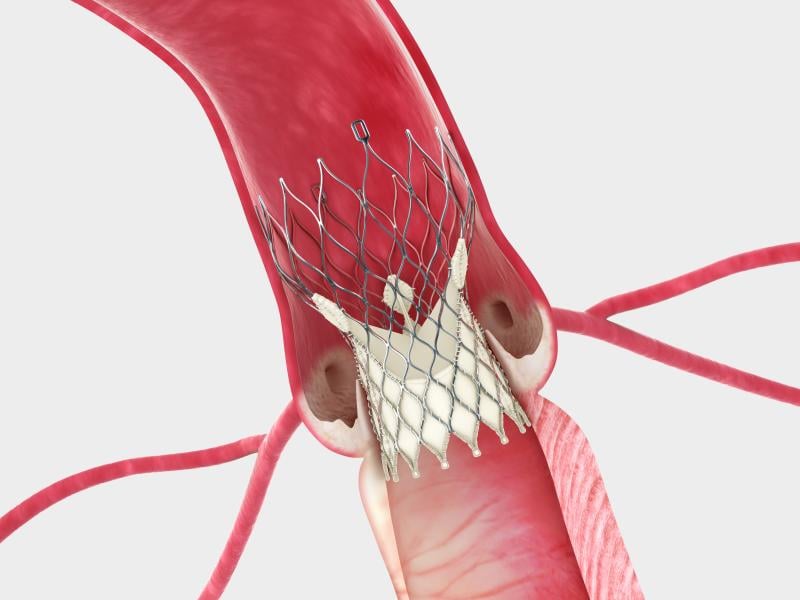
In a surprise move, Edwards Lifesciences Corp. and Medtronic reached an agreement this week to settle all outstanding patent litigation between the companies, including cases related to transcatheter heart valves. The agreement will result in the dismissal of all pending cases or appeals in courts and patent offices worldwide, and includes a provision that the parties will not litigate patent disputes with each other in the field of transcatheter valves for the eight-year duration of the agreement.

May 19, 2014 — A new study found that adding an electrocardiogram (ECG) to existing pre-participation screening of high school athletes increases the likelihood of identifying disorders associated with sudden cardiac death. The findings were presented at Heart Rhythm 2014, the Heart Rhythm Society’s (HRS) 35th annual scientific sessions.

May 19, 2014 — St. Jude Medical announced that data presented during a late-breaking clinical trial session at the 2014 annual scientific sessions of the Heart Rhythm Society (HRS) found an association between adherence to remote monitoring with the Merlin remote monitoring system and a reduction in patient mortality.


 May 22, 2014
May 22, 2014









