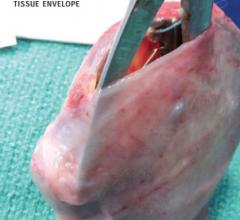
CorMatrix Cardiovascular announced that it has received U.S. Food and Drug Administration (FDA) clearance to market the CorMatrix CanGaroo ECM Envelope for use with cardiac implantable electronic devices (CIED’s) including pacemakers and implantable cardioverter defibrillators (ICD’s).
September 3, 2014 — Jobst Vascular Institute is conducting pioneering research that looks at using stents to treat blocked veins. The Cook Medical VIVO clinical research study aims to determine the safety and effectiveness of the Zilver vena venous self-expanding stent in the treatment of vein obstructions.
September 3, 2014 — Teleflex announced a newly published clinical study demonstrating the accuracy of the Arrow VPS G4 vascular positioning system, with placements of peripherally inserted central catheters (PICCs), can eliminate the use of confirmatory chest X-rays.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The easy-to-use online resource mHealth Roadmap includes information on implementation guidelines for mobile and mHealth strategies and applications. Topics covered in the Healthcare Information and Management Systems Society (HIMSS) mHealth Roadmap include:
Philips has launched Affiniti. The new ultrasound system made its debut at the European Society of Cardiology (ESC) Congress 2014 in Barcelona.
September 2, 2014 — Boston Scientific has released the primary endpoint results from its NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) clinical trial, the first and only randomized sham-controlled clinical trial investigating vagus nerve stimulation (VNS) for the treatment of heart failure (HF) patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 2, 2014 — Renal denervation reduces recurrent atrial fibrillation (AF) when performed with pulmonary vein isolation ablation in patients with AF and hypertension, according to research presented at the European Society of Cardiology (ESC) Congress by Alexander Romanov, M.D., from Russia.
Janssen Research & Development, LLC and its development partner, Bayer HealthCare, announced results from the X-VeRT trial, demonstrating once-daily rivaroxaban may be an alternative to vitamin K antagonists in treating and reducing the risk of blood clots in non-valvular atrial fibrillation patients undergoing elective cardioversion, a common medical procedure to reset the heartbeat back to a regular rhythm.
John Muir Health's Concord medical center is the first hospital in Northern California and one of just 20 hospitals in the nation to begin treating patients who suffer from atrial fibrillation (AFib), the most common type of heart arrhythmia, with a new procedure that features the FDA cleared FIRMap Catheter.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The high demand for qualified health IT professionals continues, as revealed in the 2014 HIMSS Workplace Study, conducted by HIMSS Analytics. The study shows more than 84 percent of survey respondents reported their organization hired at least one staff member in the past year, a finding consistent with the 2013 survey (86 percent). With hiring in 2014 expected to continue at the 2013 pace, 82 percent of survey respondents planned to hire at least one full-time employee (FTE) in the next 12 months, a slight increase from the 79 percent of respondents planning the same in 2013.
August 29, 2014 — Nuance Communications announced it has achieved an industry-first milestone in sharing 3 billion medical images through the Nuance PowerShare Network. This reflects steady growth of approximately 30 percent each year as the technology gains more widespread adoption in hospitals and facilities securely sharing patient medical images using the cloud.
August 29, 2014 — Direct Flow Medical announced it received CE mark for a 23 mm valve as part of its Direct Flow Medical transcatheter aortic valve system, expanding the patient population that can be treated with this technology. The company also announced receipt of the CE mark for implantation of all its valves without the use of contrast media, protecting patients from kidney injury during transcatheter aortic valve implantation (TAVI).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 29, 2014 — Cook Medical recalled 696 of its CloverSnare four-loop vascular retrieval snare devices, model number VRS-6.0-9.0. The device was recalled because of a potential for the loop to separate from the shaft, resulting in loss of device function, potential for embolization of snare fragments and the potential need for intervention to retrieve the separated snare.
August 29, 2014 — CardiacAssist announced it has received a Class 2 medical device license from Health Canada for its new Protek Duo veno-venous cannula. The Protek Duo is licensed for use as a single cannula for both venous drainage and reinfusion of blood via an internal jugular vein during extracorporeal life support procedures.

August 29, 2014 — MedAxiom Consulting announced the release of its 2014 Provider Compensation & Productivity Report, based on 2013 data submitted by 134 cardiology programs representing 2,554 cardiologists across the country. Of these responding groups, 97 of them are integrated and 37 remain private.

 September 03, 2014
September 03, 2014















