Shockwave Medical announced that the company will present clinical results from DISRUPT PAD, a single-arm multicenter study evaluating the safety and utility of Lithoplasty balloon catheters for the treatment of peripheral artery disease (PAD), at the Vascular Interventional Advances (VIVA) Annual Conference taking place Nov. 4-7 in Las Vegas.
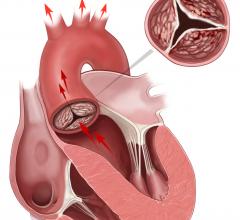
Genetic predisposition to elevated low-density lipoprotein cholesterol (LDL-C) was associated with aortic valve calcium and narrowing of the aortic valve, findings that support a causal association between LDL-C and aortic valve disease, according to a study appearing in JAMA.
The U.S. Food and Drug Administration (FDA) has cleared the Abbott family of Xience everolimus-eluting coronary stents for the additional indication to treat coronary chronic total occlusions (CTOs).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Siemens Healthcare announces the availability of the Acuson X600, a cost-effective new ultrasound system with advanced technologies migrated from premium Siemens systems and workflow efficiencies for clinical applications in general imaging, obstetrics and gynecology, and cardiology
A diverse group of uncommon congenital heart defects and genetic conditions may put a child in danger of sudden cardiac arrest (SCA), and at least two-thirds of these defects and conditions can be identified by an electrocardiogram (ECG), the test that analyzes the heart's electrical function. Much of the current debate centers on the practicalities and cost of universal ECG screening. Two clinicians offer opposing commentaries on the controversy in the August 19 issue of Circulation, the journal of the American Heart Association.
Once a common procedure, left ventriculography’s role in assessing heart disease has evolved considerably over the decades, leading to significant variations in use. A new expert consensus statement e-published by the Society for Cardiovascular Angiography and Interventions (SCAI) recommends optimal uses for left ventriculography, with the goal of more standardized use of the test and improved quality of care for cardiac patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Siemens Healthcare announces the availability of the Acuson X600 – a new ultrasound system with advanced technologies migrated from premium Siemens systems and workflow efficiencies for clinical applications in general imaging, obstetrics and gynecology and cardiology.
The hospitals of UnityPoint Health — Des Moines offering adult cardiac services recently began using analytics software by Lumedx. The software offers flexible reports based on wide range of granular data.

There are several hemostasis devices that can help cath labs improve efficiency by reducing nursing time in the recovery ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

For any cardiology department looking to upgrade or replace its cardiovascular picture archiving and communications system (cardiac PACS), or cardiovascular information system (CVIS), the main questions that should be asked are related to integration and interoperability. These are key to leveraging health IT investments to achieve improved efficiency, help cut costs and accommodate increased patient volume.
The desire to guide product development and remain on the leading edge of cardiac care led Shannon to become a beta testing site for McKesson Cardiology 13.1, which extends remote access with the ability for physicians to view ECG waveforms and results on iPads.
The key take away messages from the 26th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium were bioresorable stents and transcatheter valve technologies will likely become primary interventional tools in the near future.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Gammex 464 CT phantom better indicates the scanner's performance with a phantom that more accurately mimics a torso. The optional Gammex 464-Ring torso adapter permits the use of the Gammex 464 Accreditation in this type of application
The American College of Cardiology announced 35 selected hospitals that are pioneering a team approach to keep patients healthy and at home following admission for heart attack or heart failure. The hospitals from across the country are the first participants in the ACC Patient Navigator Program, which is the first program of its kind in cardiology and supports national efforts to reduce unnecessary patient readmissions.
Metro Health (Michigan) cardiovascular specialist Jihad Mustapha, M.D., is one of the first physicians in the United States to use a new medical device to treat peripheral artery disease (PAD).

 November 04, 2014
November 04, 2014