Adding the HeartFlow FFRCT Analysis to a standard coronary computed tomography angiogram (cCTA) may change the course of treatment in more than one-third of patients with coronary artery disease. This conclusion was discussed in a study presented at the EuroPCR 2015 conference.
A study presented at Heart Rhythm 2015 found that botulinum toxin (botox) injections into epicardial fat pads during coronary artery bypass graft (CABG) surgery can be beneficial for the patient. The injection not only reduces the incidence of post-operative atrial fibrillation (AF), but also provides substantial AF suppression after one year.
New data from St. Jude Medical found that patients with cardiac devices who use remote monitoring have significantly fewer hospitalizations and lower overall healthcare costs than patients who do not. The data was presented during a late-breaking clinical trial session during Heart Rhythm 2015, the Heart Rhythm Society’s annual scientific sessions. The findings were a result of a five-year study, one of the largest to date on remote monitoring technologies.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Chest pain sends more than 7 million Americans to the emergency department each year. About half of them are admitted to the hospital for further observation, testing or treatment. Now, emergency medicine physicians at The Ohio State University Wexner Medical Center and Mount Carmel Health System believe that number can be significantly reduced.
A new epicardial pacing lead has been cleared by the U.S. Food and Drug Adminitysration (FDA) as an option for the implant of a pacemaker, defibrillator, or cardiac resynchronization therapy (CRT) device. The lead is indicated when other types of leads cannot be implanted. Examples include patients who have small veins, congenital heart disease, abnormalities of the tricuspid valve or when other leads are already in place, preventing additional leads in the veins.
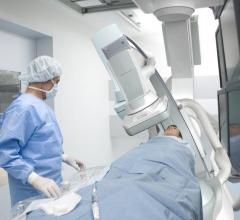
Shimadzu Medical Systems USA announced the first U.S. West Coast installation of its Trinias C-12 high performance “crossover” system at Kalispell Regional Medical Center in Kalispell, Montana. Shimadzu dealer/partner Core Medical Imaging Inc. of Kenmore, Washington, completed the installation of the angiographic system, which went into operation at the medical center in late April.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Edwards Lifesciences announced it has temporarily halted its clinical trial of the Fortis mitral transcatheter heart valve. The company said that in consultation with trial investigators, it was decided to voluntarily implement a temporary pause on enrollment in its Fortis clinical program because of evidence of valve thrombosis. The company said the issue warrants additional investigation before restarting enrollment.
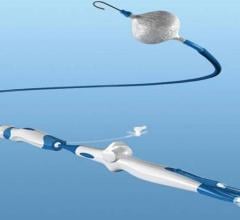
Abbott announced that it has received CE Mark for the latest advancement of its Absorb stent system, called Absorb GT1. Absorb GT1 combines a fully dissolving stent with a next-generation delivery catheter to help doctors treat people with heart disease. Built upon three generations of delivery catheter innovations, Absorb GT1 refers to the GlideTrack catheter, Abbott’s advanced stent delivery system, which is designed to make it easier for doctors to access and treat diseased vessels in people with coronary artery disease (CAD). The GlideTrack catheter incorporates several design and technology changes that have the potential to improve deliverability and performance.
Medtronic plc announced the Arctic Front Advance ST Cryoablation Catheter has received U.S. Food and Drug Administration (FDA) approval for the treatment of patients with drug refractory, recurrent, symptomatic paroxysmal atrial fibrillation. In Europe, where the Cryoballoon has a broader indication, Arctic Front Advance ST Cryoballoon has received CE Mark for the treatment of patients with atrial fibrillation.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A new service called MyChoiceMD is preparing to launch in Colorado that aims to revolutionize the way patients find, pay for, schedule and track their routine medical care. The service has been incubating in Northern Colorado and has completed a 10-month pilot in Cheyenne under the name Galen. It is targeted at the large population of self-pay patients that includes both insured and uninsured patients.
A risk assessment algorithm combining clinical risk factors and platelet function test results may help interventional cardiologists better identify patients who stand to benefit from intensive antiplatelet medication after percutaneous coronary intervention (PCI). This assessment is according to results of the TRIAGE study, presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2015 Scientific Sessions in San Diego.
New research reports significant differences between men and women with atrial fibrillation (AF) and the safety of intense physical activity. The study found that both moderate and vigorous levels of exercise are safe for women living with AF. However, vigorous levels of exercise are associated with an increased risk of AF in men. The research, analyzing data from a large-scale or robust patient population of nearly 380,000 patients, was presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Patients with a high-risk paclitaxel drug-eluting stent given the shorter-acting antiplatelet drug cilostazol prior to a surgical procedure safely transitioned off of dual antiplatelet therapy (DAPT) to reduce bleeding risk during their operation. These new findings from the OUTSIDE START study were presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2015 Scientific Sessions in San Diego.
A largest-of-its kind study has found that women who experience menopause at a younger age are at a decreased risk of atrial fibrillation (AF). The study followed nearly 18,000 women and revealed that women experiencing menopause younger than 44 years had a significantly lower risk of AF than women entering menopause between the ages of 44-50. The research was presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions.
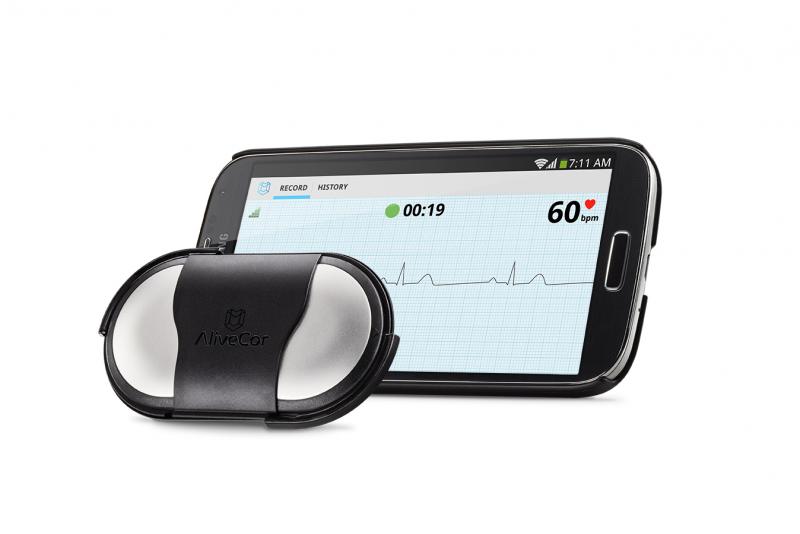
A new, large-scale study has found adding an electrocardiogram (ECG) to the latest generation of body-worn sensors accurately detects atrial fibrillation (AF) and significantly increases awareness of heart rate and behavior. Body-worn or wearable wireless sensors are increasingly being used to help collect health-related information that can be shared with a doctor through a smartphone application. The study results show that by using ECG sensors with a smartphone application, the general adult population can efficiently track their heart rate data. The new findings were presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions.

 May 20, 2015
May 20, 2015