Boston Scientific reported positive, long-term data from the EVOLVE Trial of the Synergy everolimus-eluting bioabsorbable polymer platinum chromium coronary stent system, with no new major adverse cardiac events reported between years three and four. The study results were presented at EuroPCR 2015 by Prof. Ian Meredith, director of MonashHeart, at Monash Medical Centre in Melbourne, Australia.
New clinical data from two different studies show the IN.PACT Admiral drug-coated balloon from Medtronic plc successfully treated long lesions in the superficial femoral and popliteal arteries. The data was presented at EuroPCR 2015 during the “Hot line” session on “Peripheral interventions.”
St. Jude Medical Inc. announced new results from two clinical studies further supporting the use of its fractional flow reserve (FFR) technology to optimize percutaneous coronary intervention (PCI) procedures. The studies — a 15-year follow-up to the DEFER study and primary results from the CONTRAST study — were presented during hotline sessions at EuroPCR 2015. Combined, the studies contribute to the growing body of evidence supporting FFR as a valuable and important decision-making tool for physicians.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Direct Flow Medical Inc. announced positive two-year data from the DISCOVER CE Mark Trial studying its Direct Flow Medical Transcatheter Aortic Valve System at the EuroPCR conference in Paris, France. Demonstrating excellent patient outcomes with few complications, the two-year data were presented by Antonio Colombo, M.D., Ph.D., from the Ospedale San Raffaele in Milan, Italy.
PHS Technologies Group LLC, a unit of PACSHealth LLC announced that Dell Healthcare and Life Sciences will become a marketing, distribution and hosting partner for its DoseMonitor OnLine software solution.
The Phase C results of the ProMRI Clinical Study were presented at the late-breaking clinical trial session of the Heart Rhythm Society (HRS) 2015 meeting.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
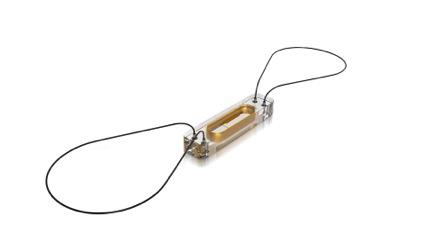
Important new data presented during the Heart Rhythm Society's (HRS) 36th annual Scientific Session supporting improved outcomes and cost-effectiveness of the CardioMEMS HF System for the management of Class III heart failure patients.
Biotronik announced that since the launch of the ProMRI Eluna pacemaker system in late March 2015, 60 percent of pacing devices sold by the company are approved for use during magnetic resonance imaging (MRI) scans. The announcement was made at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions, May 13-16 in Boston.
The Heart Rhythm Society (HRS) has released a first-of-its-kind expert consensus statement on three specific cardiovascular disorders that also involve the autonomic nervous system. The 2015 Heart Rhythm Society Expert Consensus Statement on the Diagnosis and Treatment of Postural Tachycardia Syndrome, Inappropriate Sinus Tachycardia, and Vasovagal Syncope was written by an international group of experts and published online in HeartRhythm Journal, the official journal of HRS. The expert consensus statement was presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Janssen Pharmaceuticals Inc. and its development partner Bayer HealthCare announced results from the VENTURE-AF trial. The study explored the potential of once-daily Xarelto (rivaroxaban) as an alternative to vitamin K antagonists (VKA), used to reduce the risk of blood clots, in people with non-valvular atrial fibrillation (NVAF) undergoing catheter ablation, a frequently used interventional procedure to remove abnormal tissue in the heart that is causing the irregular heartbeat. The results were presented at Heart Rhythm 2015, the Heart Rhythm Society's 36th annual scientific sessions, and published in the European Heart Journal.
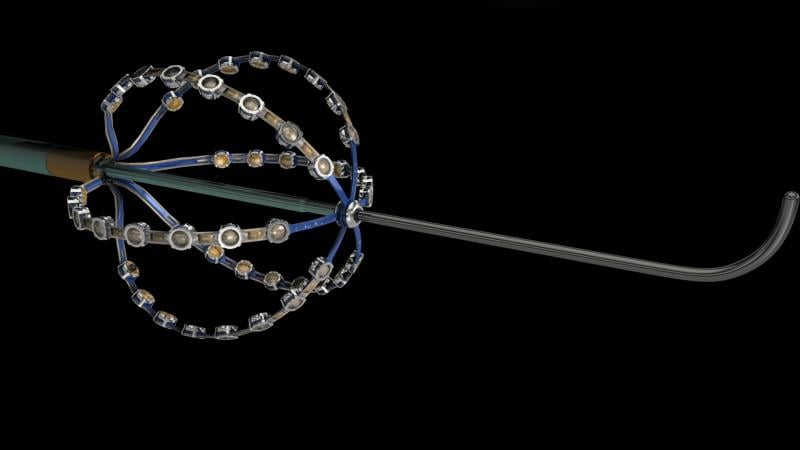
A preliminary study of a new electrophysiology (EP) mapping system using a specialized intracardiac ultrasound basket catheter for electro-mapping showed it is four times higher resolution than standard voltage mapping. Researchers also said the new mapping modality represents the electromapping data on computed tomography (CT) quality anatomy. The data suggest it may open the possibility to map irregular arrhythmias with more precision. The data was presented at Heart Rhythm Society’s 2015 meeting.
Medtronic plc announced that its Tyrx Antibacterial Envelope reduces major cardiac device site infections by 80 percent up to 12 months after implantation. These data were presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions in Boston.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Amaranth Medical announced plans to report clinical results from the company's ongoing trial of its second-generation Fortitude sirolimus-eluting bioresorbable scaffold (BRS) at EuroPCR 2015. Juan F. Granada, M.D., executive director and chief innovation officer of the CRF-Skirball Center for Innovation (SCI), will present these updates.

The first-in-human trial of a new miniaturized leadless pacemaker implanted directly inside the heart found the Medtronic Micra transcatheter pacing system (TPS) safe and effective in patients with a slow heart rhythm. Early performance results from the international Micra Transcatheter Pacing Study were presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions, May 13-16 in Boston.
Clinical trial results showed that full-body magnetic resonance imaging (MRI) scans do not affect the Medtronic Evera MRI SureScan implantable cardioverter defibrillator’s (ICD) ability to detect potentially lethal heart rhythms and deliver life-saving therapy. Data were presented during a late-breaking clinical trial session at Heart Rhythm 2015


 May 22, 2015
May 22, 2015











