Results from the LEADERS FREE trial found that a polymer-free drug-coated stent (DCS) was superior to a bare-metal stent (BMS) in high-bleeding-risk patients treated with one month of dual antiplatelet therapy (DAPT). It was the first randomized clinical trial dedicated to this particular patient population.
Results from the ISAR-DESIRE 4 trial indicate that use of a scoring balloon plus a paclitaxel-coated balloon (PCB) was angiographically superior to a paclitaxel-coated balloon alone for the treatment of restenosis within limus-eluting stents.
Direct Flow Medical Inc. announced one-year outcomes from the DISCOVER post-market study that demonstrate excellent real-world results for the Direct Flow Medical Transcatheter Aortic Valve System. The data were presented last week at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting by Federico De Marco, M.D., from the Policlinico San Donato in Milan, Italy.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

For the first time, cardiopulmonary resuscitation (CPR) guidelines issued by the American Heart Association (AHA) recommend communities consider using social media and mobile app technology to alert CPR responders when someone nearby suffers sudden cardiac arrest. The new guidelines cite studies that show emerging mobile technologies can result in a “higher rate of bystander-initiated CPR.”
Bracco Diagnostics Inc. announced that Lumason was approved by the Centers for Medicare and Medicaid Services (CMS) for pass-through status under the Hospital Outpatient Prospective Payment System (HOPPS). Lumason is an ultrasound contrast agent indicated for use in adults with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border.
A pair of studies presented at the 27th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium showed no late benefit of thrombus aspiration during percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Results from the BRAVO 3 trial found that bivalirudin did not significantly reduce major bleeding rates at 48 hours or adverse events at 30 days compared to heparin in high-risk patients undergoing transcatheter aortic valve replacement (TAVR).
October 19, 2015 — Vascular Interventional Advances (VIVA) Physicians, a not-for-profit organization dedicated to ...
The U.S. Food and Drug Administration (FDA) granted accelerated approval to Praxbind (idarucizumab) for use in patients who are taking the anticoagulant Pradaxa (dabigatran) during emergency situations when there is a need to reverse Pradaxa’s blood-thinning effects.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
There were several overarching technology trends seen at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual ...
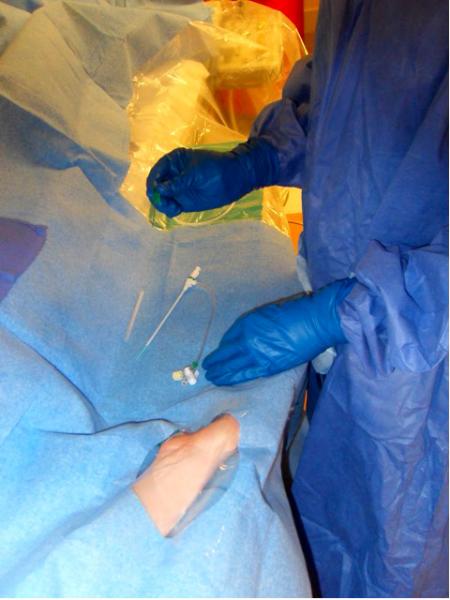
Results from a prospective randomized trial of transradial (TRI) versus transfemoral (TFO) access in patients undergoing percutaneous coronary intervention found that TRI was non-inferior in terms of clinical effectiveness at one year and superior in reducing major bleeding complications at seven days compared to TFI.

New results from a multicenter, prospective study show that assessing fractional flow reserve estimated with computed tomography (FFR-CT) may reduce costs in selected symptomatic patients with suspected coronary artery disease (CAD).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Two-year results from the IN.PACT SFA study found the use of a drug-coated balloon was superior to conventional percutaneous transluminal angioplasty (PTA) in treating patients with superficial femoral artery (SFA) disease.
The main pumping chamber of the heart ages differently in men and women, according to a new magnetic resonance imaging (MRI) study published online in the journal Radiology. Researchers said the findings may support different treatment approaches for men and women with heart disease.

Long-term study results from the RESPECT trial found that closing a patent foramen ovale (PFO) with an Amplatzer PFO Occluder was superior to medical management in the prevention of recurrent cryptogenic stroke in patients who had previously experienced one.

 October 22, 2015
October 22, 2015