St. Jude Medical Inc. announced the company has received CE Mark approval for the HeartMate 3 Left Ventricular Assist System (LVAS). HeartMate 3 is a cardiac support option for advanced heart failure patients who are awaiting transplantation, are not candidates for heart transplantation or are in myocardial recovery.
Stereotaxis Inc. announced the worldwide launch of Respiratory Compensation, a new software feature of the company’s Niobe remote magnetic navigation system.
A large observational registry found that initial aortic valve replacement (AVR) in asymptomatic patients with severe aortic stenosis (AS) was associated with a lower risk of mortality and heart failure hospitalization compared with a conservative treatment strategy.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Results from the multicenter, prospective, randomized PANDA III trial indicate that the BuMA sirolimus-eluting stent (SES) was non-inferior to the Excel SES for the primary endpoint of target lesion failure at one year. The trial specifically examined whether the rate of drug elution and polymer absorption affects the clinical outcomes of two bioresorbable polymer-based drug-eluting stents (DES).
OrbusNeich announced the presentation of the one-year clinical outcomes from the 1,000-patient REMEDEE Registry at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) meeting, Oct. 11-15 in San Francisco. Data was presented by Robbert de Winter, M.D., Ph.D., of the Academic Medical Center, Amsterdam and principal investigator of the study.
Medinol announced in September the completion of enrollment in its BIONICS trial to evaluate the safety and effectiveness of a new coronary stent system, the first-ever elastomeric drug eluting stent (eDES).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
CardioKinetix Inc. announced that it has received regulatory approval for the Parachute system in South Korea by the Korean Ministry of Food and Drug Safety (MFDS).
Results of the TUXEDO trial, designed to compare two types of stents in diabetic patients, found paclitaxel-eluting stents failed to meet non-inferiority criterion compared to everolimus-eluting stents for the primary endpoint of target-vessel failure at one year.
A randomized trial found that ranolazine did not reduce the composite rate of ischemia-driven revascularization or hospitalization in patients with a history of chronic angina who had residual unrevascularized coronary artery disease after percutaneous coronary intervention (PCI).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medtronic plc released the first CoreValve transcatheter aortic valve replacement (TAVR) system outcomes data using CoreValve data from The Society of Thoracic Surgeons and American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) Registry. The data show that everyday clinical experience from 6,160 CoreValve patients treated by a wide variety of heart team implanters replicates the excellent outcomes achieved in robust clinical trials. The primary outcomes of all-cause mortality and stroke in the STS/ACC TVT Registry were numerically similar to the findings in the CoreValve U.S. Pivotal Trial, which demonstrated statistical superiority to surgical aortic valve replacement in high-risk patients.
Results from the LEADERS FREE trial found that a polymer-free drug-coated stent (DCS) was superior to a bare-metal stent (BMS) in high-bleeding-risk patients treated with one month of dual antiplatelet therapy (DAPT). It was the first randomized clinical trial dedicated to this particular patient population.
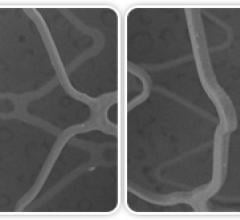
Results from the ISAR-DESIRE 4 trial indicate that use of a scoring balloon plus a paclitaxel-coated balloon (PCB) was angiographically superior to a paclitaxel-coated balloon alone for the treatment of restenosis within limus-eluting stents.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Direct Flow Medical Inc. announced one-year outcomes from the DISCOVER post-market study that demonstrate excellent real-world results for the Direct Flow Medical Transcatheter Aortic Valve System. The data were presented last week at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting by Federico De Marco, M.D., from the Policlinico San Donato in Milan, Italy.

For the first time, cardiopulmonary resuscitation (CPR) guidelines issued by the American Heart Association (AHA) recommend communities consider using social media and mobile app technology to alert CPR responders when someone nearby suffers sudden cardiac arrest. The new guidelines cite studies that show emerging mobile technologies can result in a “higher rate of bystander-initiated CPR.”
Bracco Diagnostics Inc. announced that Lumason was approved by the Centers for Medicare and Medicaid Services (CMS) for pass-through status under the Hospital Outpatient Prospective Payment System (HOPPS). Lumason is an ultrasound contrast agent indicated for use in adults with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border.


 October 28, 2015
October 28, 2015