Biointegrated sensors for long-term, continuous tracking of body chemistry may make health and disease monitoring as easy as turning on your smartphone. Unveiled by Profusa Inc., of South San Francisco, Calif., tiny bioengineered biosensors will soon enable real-time detection of our body's unique chemistry, providing actionable, medical-grade data for personal and medical use for as long as two years at a time. The company's technology and vision were presented at the CES Digital Health Summit, Jan. 6-9 in Las Vegas.
Teleflex Inc. has acquired privately held Nostix LLC, developer of innovative tip confirmation systems that are used to increase the accuracy of vascular access device placement.
Patients between the ages of 40 and 70 who undergo aortic valve replacement (AVR) may fare better with tissue-based valves than metal-based valves, according to a review article posted online by The Annals of Thoracic Surgery.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
CardioKinetix Inc. announced that it has enrolled more than half of the subjects in its pivotal United States trial, PARACHUTE IV. The trial is evaluating the Parachute device for the treatment of patients suffering from heart failure, a highly debilitating condition.
The Centers for Medicare and Medicaid Services (CMS) announced Monday that it will be ending the Meaningful Use program that was meant to encourage the adoption of electronic medical records (EMR).

January 15, 2016 — The U.S. Food and Drug Administration (FDA) has given its approval for an expanded indication study ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 14, 2016 — Cornell biomedical engineers have discovered natural triggers that could reduce the chance of life ...
Penumbra Inc. announced the U.S. launch of its new POD Packing Coil, designed as a complementary device for Penumbra’s Ruby and POD (Penumbra Occlusion Device) embolization products.
The American Medical Association (AMA) announced that it is investing $15M to become founding partner of a healthcare innovation company — Health2047 Inc.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Ionizing radiation can save lives, but it can also cause serious harm. Getting the right measurement of exposure to ionizing radiation in any situation is at the heart of an ISO standard that has recently been updated.
Even though brain natriuretic peptide (BNP)/pro-BNP and troponin biomarkers are the gold standard for cardiac disease diagnosis, end users will soon see additional alternative biomarkers entering the market, according to analysis from Frost & Sullivan.

Beyond measuring blood flow, pressure, oxygen levels and other vital signs in the cardiac catheterization lab, current ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
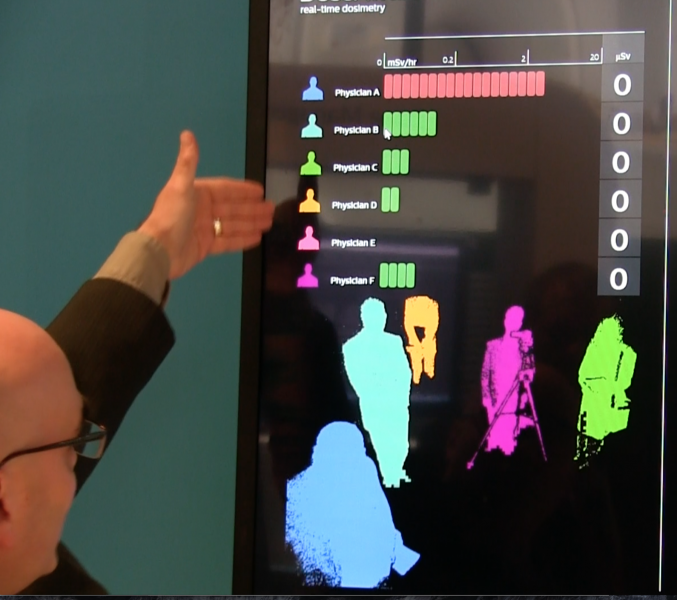
Each year radiology vendors use the Radiological Society of North America (RSNA) meeting as a springboard to unveil ...
NuCryo Vascular LLC announced that they have received U.S. Food and Drug Administration (FDA) 510(k) clearance on the next-generation reusable cryoplasty inflation device.
Consumers from California, Colorado and Wisconsin filed a nationwide class action lawsuit against Fitbit Inc. alleging the company’s wrist-based “Charge HR” and “Surge” heart rate monitors do not and cannot consistently record accurate heart rates during the intense physical activity for which Fitbit expressly markets the devices.


 January 15, 2016
January 15, 2016