The Smartdop XT by Koven is a fully automated vascular testing system that enables rapid, multiple-level bilateral studies in minutes.
Direct Flow Medical Inc. announced European commercial market launch of the DirecTrack Delivery System, a next-generation delivery system for the Direct Flow Medical Transcatheter Aortic Valve System.

Healthcare providers will need to start preparing for the next big healthcare information technology (IT) implementation ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
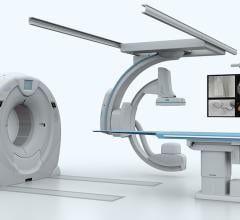
Toshiba America Medical Systems Inc. announced it would demonstrate the following diagnostic imaging technologies at this year’s American College of Cardiology 2016 Annual Scientific Session, April 2-4 in Chicago:
Corindus Vascular Robotics Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the CorPath System for use in peripheral vascular interventions.
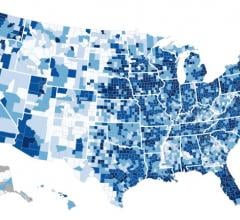
The Centers for Medicare & Medicaid Services Office of Minority Health (CMS OMH) released a new interactive map to increase understanding of geographic disparities in chronic disease among Medicare beneficiaries.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
St. Jude Medical Inc. announced CE Mark approval and launch of three new Quartet left ventricular (LV) leads, offering additional electrode spacing options and one new lead shape.
NeoChord Inc. announced that David H. Adams, M.D., and Michael A. Borger, M.D., Ph.D., will serve as national co-principal investigators of the company’s U.S. pivotal trial scheduled to commence later this year.

A person’s healthcare records contain a wealth of sensitive information. This does not involve just their medical ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
AliveCor, Inc., the leader in FDA-cleared electrocardiogram (ECG) technology for mobile devices, announced today the ...
Merge Healthcare, an IBM company, offers award-winning cardiology and hemodynamics solutions that provide a complete ...
Civco Medical Solutions announced that it has acquired PCI Medical, a market leader in high-level disinfection products for ultrasound transducers. The acquisition equips Civco with a portfolio of hardware and consumables for cost-effective infection control compliance in the ultrasound transducer reprocessing market.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Conventional ultrasound systems too often yield non-diagnostic exams. Recognizing this, GE Healthcare has developed an ...
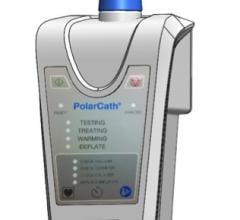
NuCryo Vascular LLC announced the launch of the Next Generation Cryoplasty Inflation device. The device received U.S. Food and Drug Administration (FDA) 510(k) clearance in late 2015 and works in conjunction with the PolarCath Balloon Dilatation System.
V-Wave Ltd, maker of an investigational interatrial shunt device for patients with advanced heart failure (HF), announced the results of its first human implants were published in The Lancet.


 March 30, 2016
March 30, 2016