Healthcare reform efforts of recent years have challenged the industry to reduce the costs of healthcare for both ...
The American Society of Echocardiography (ASE) will host its 27th Annual Scientific Sessions, June 10-14, 2016, at the Washington State Convention Center in Seattle.
BioVentrix Inc. announced that it has received a U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval to initiate its pivotal clinical trial, named ALIVE (American Less Invasive Ventricular Enhancement).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Biotronik announced the launch of CardioMessenger Smart in the United States. CardioMessenger Smart is a portable monitoring device, about the size of a modern smartphone, that keeps pacemaker, implantable cardioverter defibrillator (ICD) and insertable cardiac monitor (ICM) patients connected to their physician remotely, enabling more efficient care management anywhere in the world.
A person is admitted to the hospital with a stroke, but not much is known about whether or not that patient will undergo neuroimaging.
In a recent study, the use of a stent to repair pulmonary artery stenosis in children and adults with congenital heart disease was successful in the majority of patients, but many also experienced serious complications. The study was published in March in the Journal of the American College of Cardiology.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
MIM Software Inc. announced it has received 510(k) U.S. Food and Drug Administration (FDA) clearance to market full MIM products running on tablets through thin client technology such as Citrix.
Medic Vision Imaging Solutions Ltd. announced the U.S. Food and Drug Administration (FDA) clearance of SafeCT-29 to help healthcare facilities achieve compliance as mandated by NEMA XR-29 Smart Dose standard.
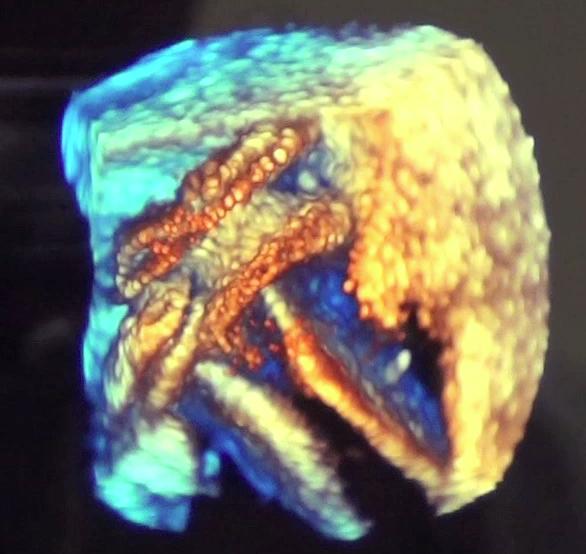
May 27, 2016 — A U.S. Food and Drug Administration (FDA) panel recommended approval of a transcatheter patent foramen ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

(Watch an October 2019 update on this article with radial adoption rates nearing 50 percent in the U.S. in the VIDEO ...
Corsens Medical Ltd. announced it has successfully completed filing of a Pre-Marketing Notification (510(k)) with the U.S. Food and Drug Administration (FDA) for its Corsens Cardiac Monitor.
May 26, 2016 — Ligand Pharmaceuticals Inc. announced the acquisition of economic rights to multiple programs owned by ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The LifeBridge Health Cardiovascular Institute, Baltimore, has launched a pilot study to evaluate the potential benefits of a wireless heart monitoring system for patients with moderate to severe heart failure.
Cancer patients at the Carti Cancer Center, Little Rock, Ark., now have access to the latest innovation in diagnostic imaging with Toshiba America Medical Systems Inc.’s Infinix 4-D CT.

May 27, 2016 — Radiation exposure to cath lab staff and physicians has seen growing concern in recent years, as the ...

 June 01, 2016
June 01, 2016