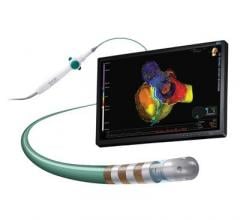
May 11, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of the company's MultiPole Pacing ...
May 11, 2017 — A new study shows that the Apple Watch's heart rate sensor, when paired with an artificial intelligence ...
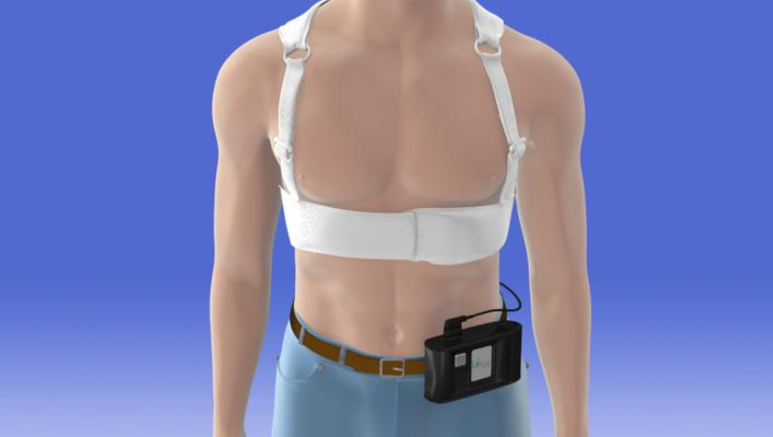
May 11, 2017 — A new study shows that the use of a wearable cardioverter defibrillator (WCD) is safe and effective in ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A large nuclear cardiology laboratory in Missouri has slashed its average radiation dose by 60 percent in eight years, according to new research presented at ICNC 2017, May 7-9 in Vienna, Austria and published in JACC: Cardiovascular Imaging.1,2 The study in over 18,000 patients showed dose reductions were achieved despite a large number of obese patients.
McKesson Imaging & Workflow Solutions, an industry leader in providing healthcare IT and imaging solutions, is pleased to announce that independent research firm KLAS has named McKesson Radiology a Category Leader in the 2017 Best in KLAS: Software & Services report for the Picture Archiving and Communications Solutions (PACS) (Community Hospital) segment, ranking number-one in the market segment.
Abbott announced CE Mark of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, developed to make it easier for physicians to more effectively treat atrial fibrillation (AF). When integrated with Abbott's EnSite Precision cardiac mapping system, physicians are able to utilize dual impedance and magnetic technologies to help more precisely model the heart. This integrated system also helps physicians determine where to apply optimal contact force (pressure) when creating a lesion during a cardiac ablation to correct a heart rhythm abnormality. The Sensor Enabled technology allows physicians to create a more detailed heart model during ablation procedures than a catheter without a sensor.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Biotronik announced the availability of the first U.S. Food and Drug Administration (FDA)-approved cardiac rhythm management (CRM) devices with technology that automatically recognizes when a patient enters a magnetic resonance imaging (MRI) environment.
Biotronik announced U.S. Food and Drug Administration (FDA) approval and the launch of Sentus ProMRI, the thinnest quadripolar left ventricular lead available in the United States. This introduction completes Biotronik's second-generation ProMRI lead portfolio, which also includes Solia ProMRI and Plexa ProMRI.
May 9, 2017 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific’s Resonate family of implantable ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 9, 2017 — The first patient has been enrolled in the U.S. Food and Drug Administration (FDA) approved prospective ...
May 9, 2017 — A manuscript by physicians from Mayo Clinic and Harvard Brigham & Women's Hospital entitled, “Initial ...
Medtronic plc recently announced three-year outcomes from the VeClose U.S. pivotal clinical trial and one-year data from the WAVES study. Both results were presented by Kathleen Gibson, M.D., of Lake Washington Vascular in Bellevue, Wash., at the 2017 Charing Cross Symposium in London. The new data demonstrate the clinical and quality of life benefits of the Medtronic VenaSeal closure system in treating patients with venous reflux disease.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
May 5, 2017 — Northwell Health recently announced that Cindy Grines, M.D., one of the nation’s pre-eminent cardiologists ...

Point-of-care (POC) coagulation analyzers that measure prothrombin time/international normalized ratio (PT/INR) on ...
A study of how policies restricting pharmaceutical promotion to physicians affect medication prescribing found physicians in academic medical centers (AMCs) prescribed fewer of the promoted drugs, and more non-promoted drugs in the same drug classes, following policy changes to restrict marketing activities at those medical centers. The analysis encompassed 16.1 million prescriptions; while the decline observed was modest in terms of percentage, proportionally small changes can represent thousands of prescriptions.


 May 11, 2017
May 11, 2017