Thirty-day results from ABSORB IV, the largest randomized everolimus-eluting bioresorbable vascular scaffold (BVS) trial to date, found BVS to be noninferior to a cobalt-chromium everolimus-eluting stent (CoCr-EES) for target lesion failure (TLF).
October 31, 2017 — The U.S. Food and Drug Administration (FDA) issued a warning to healthcare providers that interim ...
October 30, 2017 — New study results from the EXCEL trial comparing the quality of life (QoL) of patients with left main ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The American College of Cardiology, along with the American Heart Association and the Heart Rhythm Society, published new guidelines for the treatment of patients with ventricular arrhythmias and the prevention of sudden cardiac death.
October 30, 2017 — Researchers have discovered a previously unrecognized healing capacity of the heart. In a mouse model ...
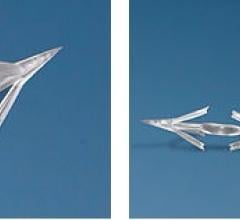
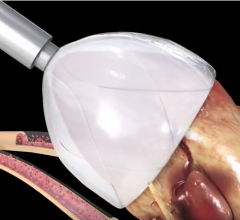
Micro Interventional Devices Inc. (MID) recently announced the second successful implantation of its MIA (Minimally Invasive Annuloplasty) technology. The patient was enrolled in the first arm of the STTAR (Study of Transcatheter Tricuspid Annular Repair) clinical trial studying the safety and efficacy of MID's MIA device designed to eliminate or greatly reduce tricuspid regurgitation.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
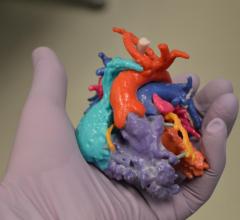
Entering their pre-market phase of development, Materialise is working with select U.S. and EU hospital partners to validate the importance of accurate 3-D modeling to help physicians plan complex transcatheter mitral valve replacement and repair (TMVR/r) procedures.
CeloNova BioSciences Inc. announced the U.S. Food and Drug Administration (FDA) approved expansion of CeloNova's ongoing clinical trial of its proprietary Cobra PzF NanoCoated Coronary Stent (NCS) with 14-day dual antiplatelet therapy (DAPT) in complex patients, such as those who are at high bleeding risk. The COBRA REDUCE trial is the world's first and only randomized control trial to assess 14-day DAPT after percutaneous coronary intervention (PCI), according to the company.
New Milford, Conn., resident Neta Awasthi is thankful to be home with his wife, Rhicha, and two teenage daughters after what they deem a miraculous recovery from a sudden, life-threatening health crisis that nearly took his life.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Adam Greenbaum, M.D., co-director of the structural heart program at Henry Ford Health System in Detroit, discusses the ...

Here is an aggregation of DAIC news, videos and articles for recent heart valve technology advances in 2017. TAVR Stands ...
CorInnova Inc. announced it has received notice of allowance of a seminal patent to protect its intellectual property associated with the world’s first minimally invasively-delivered soft robotic heart device to support heart function. The company also announced that it has become a resident at Johnson & Johnson Innovation, JLABS at the Texas Medical Center (JLABS @ TMC). This residency follows a $6.1 million funding from the Wellcome Trust, details of which have not been disclosed.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

Here is an aggregation of DAIC content for all major cardiovascular technology news and advances this past year. These topics will be discussed in sessions at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) conference in Denver, Oct. 29 - Nov. 2.
October 26, 2017 — The National Institutes of Health has awarded Detroit cardiologist Adam Greenbaum, M.D., for his ...
Corindus Vascular Robotics Inc. announced that it will incorporate the HeartFlow FFRct (fractional flow reserve-computed tomography) Analysis in a case series of robotic-assisted percutaneous coronary intervention (PCI) procedures with the CorPath GRX System to evaluate the feasibility and utility of clinical decision support.

 October 31, 2017
October 31, 2017