Siemens Healthineers will announce U.S. Food and Drug Administration (FDA) clearance of four new system features for the Biograph mCT family of positron emission tomography/computed tomography (PET/CT) systems at the 2018 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI). These features, three of which are also available on the Biograph Vision PET/CT scanner, bring advanced clinical capabilities to healthcare providers, enabling them to expand precision medicine and transform the delivery of patient care.
June 19, 2018 – Amaranth Medical offered new details about its 85-micron Defiance bioresorbable scaffold (BRS) recently ...
Clinical practice, along with guidelines and research, have shown that speckle tracking strain imaging can improve diagnostic assessment, standardize interpretation and may assist in the way patients are monitored over time to optimize care. Since 2016, there has been an investigational CPT code (0399T) available for myocardial strain echo studies, which many institutions are now reporting reimbursement. At the 2018 American Society of Echocardiography (ASE) annual meeting, June 22-26 in Nashville, Epsilon Imaging will demonstrate how imaging departments can integrate strain imaging into their echoc programs with EchoInsight.
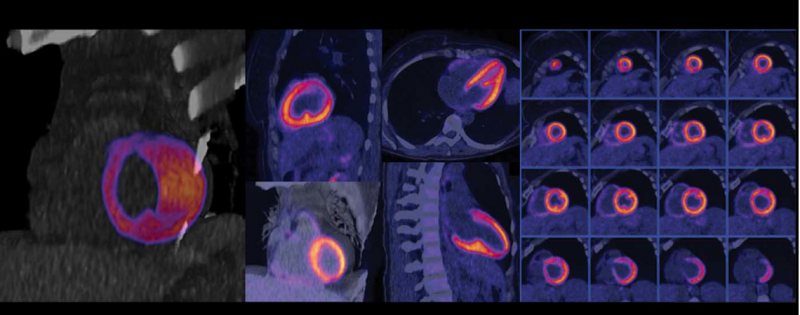
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Cardiovascular imaging artificial intelligence (AI) company Bay Labs announced its EchoMD AutoEF software received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the fully automated clip selection and calculation of left ventricular ejection fraction (EF). EF is the single most widely used metric of cardiac function and used as the basis for many clinical decisions. The EchoMD AutoEF algorithms eliminate the need to manually select views, choose the best clips and manipulate them for quantification, an often time-consuming and highly variable process.
June 18, 2018 — Heart defects are the most common type of birth defect, and can be caused by mutations in the gene CHD4 ...
3D Systems announced availability of its new On Demand Anatomical Modeling Service. This new service provides a wide range of medical professionals with access to anatomical models 3-D-printed from their 3-D digital files, enabling enhanced three-dimensional visualization for surgical planning as well as patient education.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Nuclear imaging technology for both single photon emission computed tomography (SPECT) and positron emission tomography ...
LivaNova PLC announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Memo 4D semi-rigid mitral annuloplasty ring. The company also confirmed the first implantation of the device.
Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for 200mm and 250mm lengths of the In.Pact Admiral Drug-Coated Balloon (DCB) to treat long superficial femoral artery (SFA) lesions in patients with peripheral artery disease (PAD).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A team of researchers says it has linked sensitivity to an allergen in red meat to the buildup of plaque in the arteries of the heart. While high saturated fat levels in red meat have long been known to contribute to heart disease for people in general, the new finding suggests that a subgroup of the population may be at heightened risk for a different reason – a food allergen. The study, which is supported by the National Heart, Lung and Blood Institute, part of the National Institutes of Health, appears in Arteriosclerosis, Thrombosis, and Vascular Biology (ATVB), a peer-reviewed journal of the American Heart Association.
The U.S. Food and Drug Administration (FDA) released the following two draft guidance documents: Coronary, Peripheral, and Neurovascular Guidewires - Performance Tests and Recommended Labeling; and Intravascular Catheters, Wires, and Delivery Systems with Lubricious Coatings - Labeling Considerations.
This is a 360 degree image from the Canon Aquilion One 320-slice computed tomography (CT) system installed at ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Henry Ford Hospital is one of 17 U.S. trial sites using a catheter-based procedure approved in Europe to repair a leaky mitral heart valve.
Medication management platform Medisafe released data showing that, on average, hypertensive patients increase their prescription refills by almost 10 percent after they start using the company's mobile app.
The thought of losing up to $14 a week along with personalized goal setting may have motivated ischemic heart disease patients to increase their exercise, according to a new clinical trial published in Journal of the American Heart Association, the Open Access Journal of the American Heart Association/American Stroke Association.


 June 21, 2018
June 21, 2018