A program designed to help heart attack patients with the transition from hospital to outpatient care can reduce readmissions and deaths and increase the number of patients keeping follow-up appointments, a new study suggests. Findings from the Sanger Heart and Vascular Heart Care Navigation Team study were presented at the American College of Cardiology’s Cardiovascular Summit, Feb. 14-16 in Orlando, Fla. The conference brings together top experts to discuss and review innovative, relevant cardiovascular management and leadership strategies.
DiA Imaging Analysis announced the launch of LVivo SAX, a cardiac analysis tool that helps clinicians quickly and accurately interpret ultrasound images and assess heart functionality among patients suspected of suffering from acute coronary syndrome (ACS).
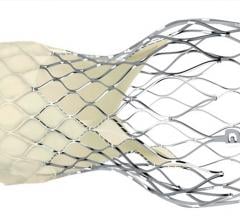
Rolling Stones frontman Mick Jagger is reportedly recovering after undergoing a transcatheter aortic valve valve replacement (TAVR) procedure at a New York hospital.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Echocardiography reporting systems are usually integrated with, or offered as an add-on module for a cardiovascular ...
Diagnostic and Interventional Cardiology (DAIC) was recently recognized as a finalist in the Jesse H. Neal Awards for ...
TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) granted premarket approval for its SuperSaturated Oxygen (SSO2) Therapy. SSO2 Therapy provides interventional cardiologists with the first and only FDA-approved treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage in heart attack patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Healthereum LLC released the beta version of the Healthereum Life Portfolio, or HELIO, a mobile application that allows patients to be rewarded digital tokens for interacting with their healthcare providers. With each action taken — “Promise to Show” for an appointment, complete quality care surveys after the visit, receive task messages from their doctors and view their current diagnoses — digital tokens are given to the patients’ “wallet” to reward them for time spent on their health using blockchain technology.
April 5, 2019 — Medical device startup HeartHero was the winner in the Innovation Challenge at the 2019 American College ...
Three years ago this week, Abiomed's Impella heart pump received U.S. Food and Drug Administration (FDA) premarket approval (PMA) for acute myocardial infarction (AMI) cardiogenic shock. At the time of Impella’s FDA PMA approval, the cardiogenic shock survival rate to explant in the Impella Quality Assurance (IQ) Database was 51 percent in the United States1. Today, Impella heart pumps, combined with the adoption of best practices that include the use of Impella pre-percutaneous coronary intervention (PCI), have contributed to a significant increase in cardiogenic shock survival and native heart recovery. New data from the IQ Database on nearly 5,000 patients treated between April 2018 and March 2019 show an increase in survival from 51 percent to 67 percent2, a relative increase of 34 percent in survival.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The U.S. Food and Drug Administration (FDA) granted Omega Medical Imaging 510(k) clearance to offer their artificial intelligence (AI)-powered region of interest (ROI) radiation exposure reduction solution FluoroShield for interventional X-ray imaging on their flat panel detector CS-series product lines.
Increasing demand for innovative diagnostic techniques, neurological disorders and increasing disease awareness are expected to drive growth of virtual reality (VR) in the healthcare market over the next several years, according to new research from Reports and Data. The report projects the market will reach a value of $6.91 billion by 2026. Advancements in the field of information technology (IT), including laptop, computer, internet connectivity and mobile applications will also be a significant factor stimulating market demand.
April 3, 2019 – The ACC.19 late-breaking landmark Evolut Low Risk Trial compared the minimally invasive Evolut ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently granted an additional indication to Bard Peripheral ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently cleared Bard Peripheral Vascular's Venovo Venous ...
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...


 April 09, 2019
April 09, 2019